Figure 1. Regulation of the AMP-activated protein kinase.
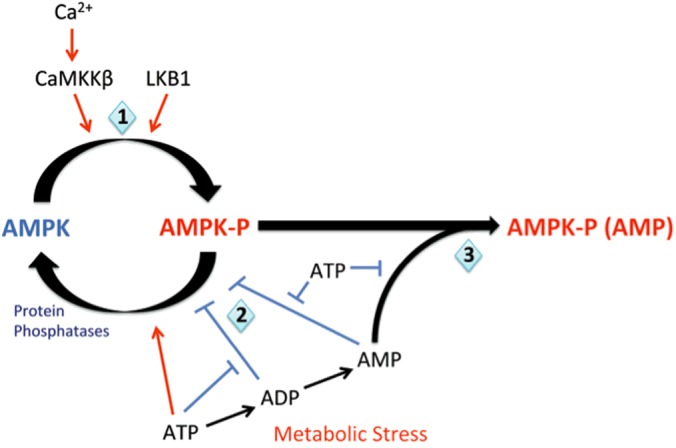
(1) AMPK is constitutively phosphorylated (AMPK-P) by LKB1. However when ATP is bound to AMPK, dephosphorylation by protein phosphatase 2C (PP2C) is promoted and AMPK remains deactivated (AMPK). Metabolic stresses, such as hypoxia, increase the AM(D)P/ATP ratio and promote displacement of ATP by AMP, and to a lesser extent by ADP, from three sites on the AMPK γ subunit. Binding of AMP or ADP to the γ subunit may promote phosphorylation by LKB1 and at the same time (2) inhibit dephosphorylation by PP2C. (3) AMP, but not ADP, binding also promotes further allosteric activation of AMPK. These three mechanisms deliver AMPK activation in response to metabolic stresses. In addition, AMPK can be activated in a Ca2+-dependent manner through CaMKK-β, which phosphorylates the same γ subunit site as LKB1. Figure adapted from [179]: Hardie, D.G., Salt, I.P., Hawley, S.A. and Davies, S.P. (1999) AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem. J. 338, 717–722.
