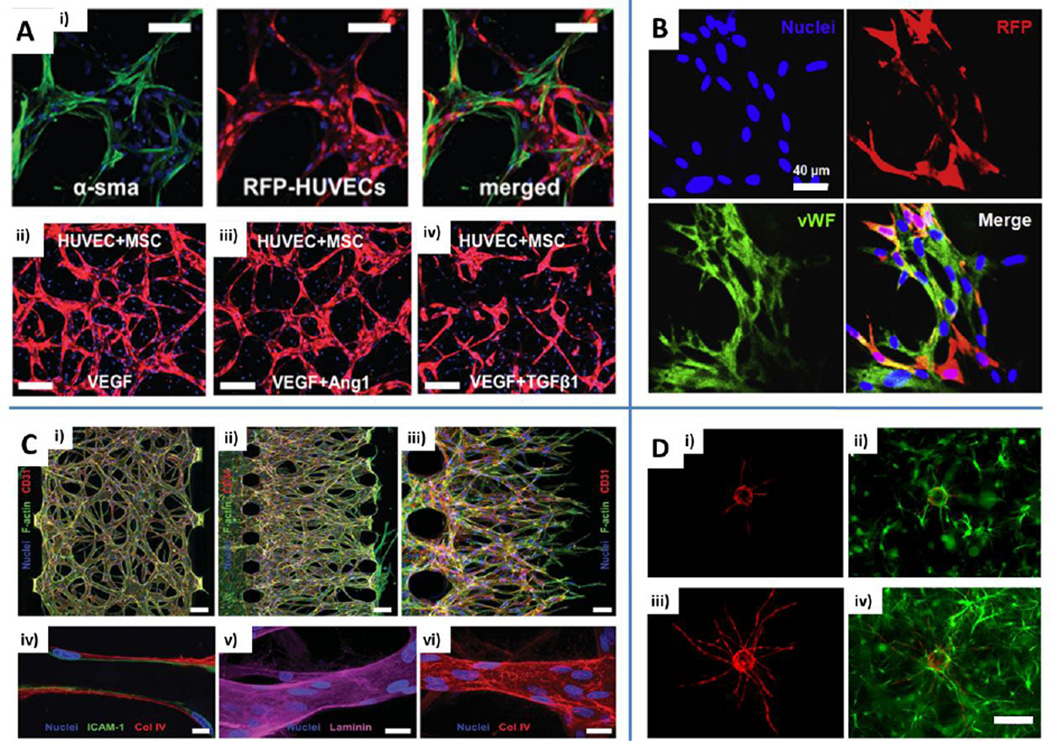
Fig. 2.
Engineering microvascular networks through vasculogenesis and angiogenesis-based techniques. A) Human umbilical vein endothelial cell (HUVEC)-human bone marrow-derived mesenchymal stem cell (MSC) co-culture demonstrating direct contact is necessary to promote MSC phenotypic transition toward mural-like cells expressing alpha smooth muscle actin (α-SMA). RFP: red fluorescent protein. Scale bars: 100 µm (i). Different microvascular network architectures obtained with the addition of vascular endothelial growth factor (VEGF), angiopoietin (Ang)-1 or transforming growth factor (TGF)-β1. Scale bars: 200 µm (ii, iii, iv). Blue: nuclei (DAPI). Adapted from Jeon et al. (2014) by permission of the Royal Society of Chemistry. B) Immunofluorescent staining of microvascular networks formed by 3D aggregates of HUVECs and RFP cord blood derived MSCs. HUVECs stained with von Willebrand factor (vWF, green). Scale bar: 40 µm. Reproduced from Chen et al. (2013) by permission of Elsevier. C) Engineered microvascular networks by means of vasculogenesis (i) or angiogenesis-based (ii) approach. Red: CD31. Green: F-actin. Tumor necrosis factor (TNF)-α stimulated vessels stained for intercellular cell adhesion molecule (ICAM)-1 (iv, green, scale bar: 10 µm). Endothelial cells secrete basic components of the basement membrane such as laminin (v, purple, scale bar: 20 µm) and collagen type IV (vi, red, scale bar: 20 µm). Blue: nuclei (Hoechst 33342). Reproduced from Kim et al. (2013) by permission of the Royal Society of Chemistry. D) HUVECs coated on microcarrier beads (i and iii, red) were cultured in fibrin gels containing bone marrow MSCs (ii and iv, green) and the sprouting process was imaged at day 3 (i and ii) and day 7 (iii and iv). Reproduced from Carrion et al. (2013) by permission of Elsevier.