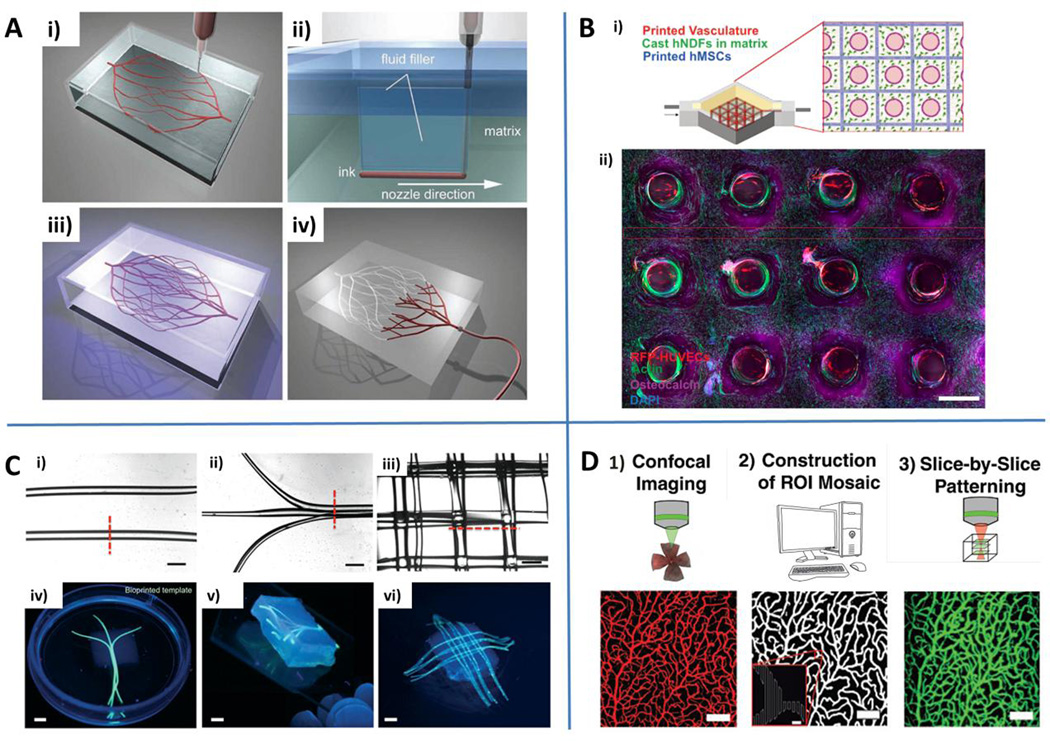
Fig. 3.
Engineering microvascular networks through bioprinting and light-based approaches. A) Omnidirectional bioprinting of 3D vascular networks. Deposition of a fugitive ink (i), filling of gaps generated by the nozzle translation during bioprinting (ii), photopolymerization (iii) and liquefaction/removal of the template to generate microvascular channels (iv). Reproduced from Wu et al. (2011) by permission of John Wiley and Sons. B) Schematic of a bioprinted multicellular tissue (i) and confocal image through a cross-section of 1 cm thick vascularized tissue cultured under perfusion for 30 days (ii). Red: HUVECs. Green: F-actin. Purple: osteocalcin. Blue: nuclei (DAPI). Reproduced by permission from Kolesky DB, Homan KA, Skylar-Scott MA and Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A. 2016, epub ahead of print. C) Gelatin methacryloyl (GelMA) hydrogels embedding bioprinted vascular networks. Linear parallel (i), planar bifurcating (ii) or 3D lattice architecture (iii) of agarose template fibers. Scale bars: 1 mm. Photographs of bioprinted templates: parallel bifurcating (iv), 3D branching template (v) and 3D lattice architecture (vi). Scale bars: 3 mm. Reproduced from Bertassoni et al. (2014) by permission of the Royal Society of Chemistry. D) Light-based vascular network patterning. Synthetic description of the main steps of the process: confocal imaging, reconstruction of cross-sectional structures, and slice-by-slice light patterning of vascular structures. Scale bar: 100 µm (5 µm for inset). Reproduced from Culver et al. (2012) by permission of John Wiley and Sons.