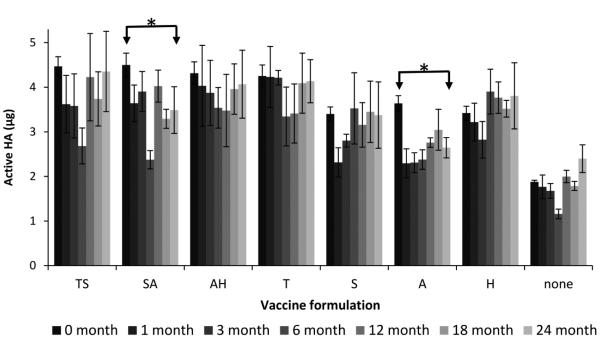
Fig. 2.
Effect of formulation on hemagglutinin (HA) activity after fabricating microneedle patches and storing with desiccant for up to 24 months at 25°C. Monovalent microneedle patches were fabricated with B/Brisbane/60/2008 influenza vaccine formulated with stabilizing excipients at a total concentration of 10% w/v in the vaccine casting solution. The two-stabilizer combinations contained equal amounts of each excipient. T = trehalose, S = sucrose, A = arginine, H = sodium heptagluconate. HA activity is shown as the mass of active HA in each patch. Asterisk (*) indicates a significant difference in vaccine activity between the initial time point and the furthest time point (Student’s t-test, p < 0.05). Data represent averages of n = 6 replicates, with standard deviation bars shown.