Abstract
The coordinated expression and activity of the iodothyronine deiodinases regulate thyroid hormone levels in hypothyroidism. Once heralded as the pathway underpinning adequate thyroid-hormone replacement therapy with levothyroxine, the role of these enzymes has come into question as they have been implicated in both an inability to normalize serum levels of tri-iodothyronine (T3) and the incomplete resolution of hypothyroid symptoms. These observations, some of which were validated in animal models of levothyroxine monotherapy, challenge the paradigm that tissue levels of T3 and thyroid-hormone signalling can be fully restored by administration of levothyroxine alone. The low serum levels of T3 observed among patients receiving levothyroxine monotherapy occur as a consequence of type 2 iodothyronine deiodinase (DIO2) in the hypothalamus being fairly insensitive to ubiquitination. In addition, residual symptoms of hypothyroidism have been linked to a prevalent polymorphism in the DIO2 gene that might be a risk factor for neurodegenerative disease. Here, we discuss how these novel findings underscore the clinical importance of iodothyronine deiodinases in hypothyroidism and how an improved understanding of these enzymes might translate to therapeutic advances in the care of millions of patients with this condition.
Introduction
Thyroid hormones are iodinated molecules produced by the thyroid gland that regulate development, growth, energy homeostasis, cardiovascular systems, musculoskeletal systems and cognitive function. Insufficient levels of the thyroid hormones tetraiodothyronine (T4) and tri-iodothyronine (T3) result in hypothyroidism, a prevalent condition that affects more than 8 million patients in the USA alone1 and 1–2% of individuals living in iodine-replete communities.2
Over the past 150 years, treatment modalities for hypothyroidism have been developed around thyroid hormone ‘replacement’ through administration of thyroid gland extracts,3 which remained the mainstay of therapy for nearly a century. However, with the discovery in 1970 that, in humans, iodothyronine deiodinases produce most of the circulating T3,4 clinical standards abruptly shifted to align with the assumption that levothyroxine monotherapy would maintain the pool of T4 and that a group of enzymes known as the iodothyronine deiodinases would provide physiologic regulation of the T3 availability to tissues.5
For the past few decades, clinicians have displayed an almost dogmatic reliance upon the ability of the iodo-thyronine deiodinases to mediate conversion of T4 to T3 and thereby regulate the availability of serum levels of T3 among patients with hypothyroidism who are treated with levothyroxine.6 However, we now understand that ~12% of all patients treated with levothyroxine are biochemically euthyroid (that is, they have normal serum levels of TSH) but continue to experience residual symptoms of hypothyroidism, including psychological7–9 and metabolic effects.10,11 This finding represents a major public-health concern given the high prevalence of hypothyroidism and the fact that not all parameters are restored by levothyroxine monotherapy. With such strong reliance on the iodothyronine deiodinases and, therefore, the efficacy of levothyroxine monotherapy, clinicians were left with few options to explain or treat these residual symptoms.
As awareness of this subpopulation of patients with hypothyroidism has improved, investigators have made advances in understanding the aetiology of this phenomenon. Serum levels of T3 might not be fully normalized among such patients owing to insufficient T4-to-T3 conversion,12 which could explain why a minority remain symptomatic despite treatment with levothyroxine. Some patients demonstrate improved well-being and a treatment preference when co-administered levothyroxine and liothyronine;13,14 however, this issue has remained contentious as the majority of clinical trials have failed to demonstrate an objective benefit of combination therapy.15,16
Differences within subgroups of patients with hypothyroidism could make them more or less responsive to levothyroxine monotherapy versus combination therapy.13 Therefore, the iodothyronine deiodinases that were once almost universally accepted as the key clinical strategy to thyroid-hormone regulation in hypothyroidism might actually cause continued symptoms among a substantial proportion of patients.
Here, we review the physiologic role of the iodothyronine deiodinases, as well as the changes that occur in hypothyroidism, with particular focus on their role in preserving thyroid hormone levels in the brain. The latest studies indicate that unique biochemical aspects of the iodothyronine deiodinases in the hypothalamus prevent normalization of serum levels of T3 in patients treated with levothyroxine monotherapy. In combination with a prevalent DIO2 polymorphism, this finding could also explain the insufficient symptomatic response experienced by an appreciable proportion of patients with hypothyroidism.
Animal models of thyroid replacement
Further insight into the regulation of iodothyronine deiodinases and tissue-specific thyroid-hormone signalling is required to develop new strategies to treat hypothyroidism. However, studies in humans are limited as they require tissue biopsy samples. Thus, animal models have been developed that provide the basis for how unique aspects of iodothyronine deiodinase regulation might affect different thyroid-hormone replacement therapies.17–20
The applicability of such models reflects the extensive similarities in thyroid physiology between humans and small rodents.21 Interspecies differences exist but their interference can be minimized by correct experimental planning. For example, the half-lives of T4 and T3 are longer in humans (~1 week and 1 day, respectively) than in rodents (~8 h and 2 h, respectively) and so blood and/or tissue sampling should be planned accordingly.21 Iodothyronine deiodinases are conserved across species at the amino acid level but they have species-specific differences in tissue distribution. Type 2 iodothyronine deiodinase (DIO2) is expressed in the human thyroid gland and heart but not in these tissues in rodents.22,23 Furthermore, DIO2 is the only activating iodothyronine deiodinase in the human brain, whereas both Dio2 and type 1 iodothyronine deiodinase (Dio1) are expressed in the rat brain.24,25
With these differences in mind, studies in thyroidectomized rats have conclusively shown that only therapy with a combination of levothyroxine and liothyronine could normalize serum and tissue levels of T3.18,19 Similar conclusions were obtained for normalization of T3-dependent biological parameters, such as lipid profile, mitochondrial content and the expression of T3-target genes, in the brains of thyroidectomized rats.17 Mouse models were also instrumental in the discovery that preserving the stability of serum concentrations of T3 is a biological priority, which is enforced even when Dio1 and/or Dio2 are genetically inactivated in all tissues26 or in specific tissues.27,28 Therefore, animal models have provided substantial advances in our understanding of thyroid-hormone replacement therapy.
Thyroid-hormone signalling
In humans, thyroid hormones are secreted into the circulation predominantly as a prohormone (T4), and only ~20% is secreted as the biologically active T3 form. T4 and T3 enter almost all cells through transporters in the plasma membrane and remain in equilibrium between plasma and cells (Figure 1). Once inside a cell, T3 diffuses into the nucleus and binds to a thyroid-hormone receptor (either TRα or TRβ) to modulate gene expression. The T3–TR complex controls expression of specific sets of genes that are responsive to T3, thus promoting T3-dependent biological effects. Tissues contain different combinations of TRα and/or TRβ as well as other transcriptional coregulators of the T3–TR complex. The net effect of T3, therefore, depends on these combinations and is highly cell and/or tissue-specific.29,30
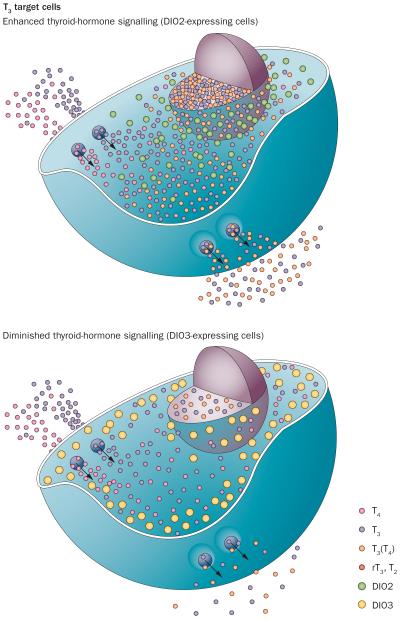
Figure 1.
Iodothyronine deiodinases modulate thyroid-hormone signalling in T3 target cells. T4 and T3 enter almost all cells via membrane transporters and are then modified in a cell-specific manner by DIOs to either enhance (DIO2) or diminish (DIO3) thyroid-hormone signalling. Consequently, the flow of T3 diffusing from the cell membrane to the nucleus is increased by T3(T4), which represents T3 generated locally from T4 via DIO2. By contrast, the DIO3 pathway decreases the flow of T3 to the nucleus by terminally inactivating T3 to T2 and T4 to rT3 (rT3, T2). DIO2 generates T3 in a cell compartment adjacent to the nucleus. By contrast, DIO3 largely resides in the periphery of the cell in the plasma membrane and early endosomes. Once inside cells, T3 can diffuse to the nucleus to modulate gene expression. Abbreviations: DIO, iodothyronine deiodinase; rT3, reverse tri-iodothyronine; T2, di-iodothyronine; T3, tri-iodothyronine; T4, tetra-iodothyronine. Courtesy of BiancoLab.org.
Studies in patients, animal models and in vitro cell models with mutations in the genes encoding TRα or TRβ have highlighted the mechanisms involved in the positive stimulation of gene transcription by T3.29 Given the free T3 concentration in the plasma (about 5 × 10−12 M) and the affinity of TRs for T3 (about 1 × 109 l/mol), the ratio of occupied to unoccupied TRs is 1:1 in most tissues. Even when unoccupied, TRs are predominantly bound to specific cis-acting elements in T3-responsive genes, such as ectonucleotide pyrophosphatase/phosphodiesterase 2 (ENPP2) and myelin basic protein (MBP). Unoccupied TRs have a high affinity for negative coregulators of transcription (also known as corepressors), which actively inhibits gene expression. Upon binding to T3, TRs lose their affinity for corepressors and gain affinity for coactivators, which triggers transcriptional activation of T3-dependent genes. Therefore, the clinical syndrome of an individual with hypothyroidism is largely the result of transcriptional repression of T3-responsive genes mediated by unoccupied TRs. The goal of thyroid-hormone replacement therapy is to provide sufficient T3 to relieve TR-mediated gene repression and to promote T3-dependent transactivation of target genes.29 The unique role of the unoccupied TRs is further illustrated by the fact that mice with a knockout for the genes encoding TRα and TRβ exhibit only a mild phenotype, mainly because no transcriptional repression occurs.29 Therefore, the intensity of thyroid-hormone signalling depends on the ratio between occupied and unoccupied TRs, which is a function of the plasma T3 concentration, presence of plasma membrane transporters, iodothyronine deiodinase activity and (ultimately) the nuclear concentration of T3.
Iodothyronine deiodinases
Iodothyronine deiodinases are small, highly homologous, integral membrane enzymes that modify thyroid-hormone signalling. They comprise a single N-terminal, transmembrane segment connected to a larger globular cytosolic domain with a selenocysteine-containing active centre embedded in a thioredoxin-like fold. The molecular structure has been modelled using hydrophobic cluster analysis31 and confirmed with supportive experimental data in the case of type 3 iodothyronine deiodinase (DIO3).32 The selenium in the active centre provides enhanced substrate affinity and a fast turnover rate for the deiodination reaction.
Both T4 and T3 can be deiodinated, a process that results in either activation of T4 (by DIO1 and DIO2) or inactivation of T4 and T3 (by DIO3). Consequently, the intracellular environment can be enriched with additional T3 supplied by DIO2 (enhancing thyroid-hormone action) or depletion of thyroid hormones by DIO3 (dampening thyroid-hormone action; Figure 1). A key property of iodothyronine deiodinases in thyroid-hormone signalling is their unique subcellular localization. DIO2 is usually retained in the endoplasmic reticulum;33,34 however, it is also closely associated with the cell nucleus,35 but not with the Golgi apparatus.33 As a result, the nuclear environment can be greatly affected by DIO2-generated T3 owing to this physical proximity. By contrast, DIO1 is located in the plasma membrane; it has low affinity for T4 and so DIO1-generated T3 rapidly diffuses from the cells and reaches the plasma without appreciably affecting nuclear concentrations of T3.34,36
DIO3 is generally anchored in the plasma membrane, where it is internalized to become part of vesicles known as early endosomes that can be recycled back to the plasma membrane.34,37 In the rat central nervous system, Dio3 is observed in dense-core vesicles of hypothalamic neuro-secretory axon varicosities with the active centre containing the C-terminus of Dio3 at the outer surface of these organelles.38 However, this peripheral distribution of Dio3 can change depending on oxygen availability. For example, after unilateral induction of ischaemia and hypoxia in the rat brain, Dio3 is found predominantly in the nucleus of the neurons in the pyramidal and granular ipsilateral layers, as well as in the hilus of the dentate gyrus of the hippocampal formation.39 In isolated mouse hippocampal neurons grown in culture and in a human neuroblastoma cell line, hypoxia redirects active DIO3 to the nucleus via the HSP40 pathway, a shuttle mechanism known to direct proteins to the nucleus. In the human neuroblastoma cell line, preventing nuclear DIO3 import by HSP40 knock-down almost doubles the thyroid-hormone-dependent glycolytic rate and quadruples the transcription of thyroid-hormone target gene ENPP2. By contrast, over-expression of HSP40 increases nuclear import of DIO3 and minimizes the effects of thyroid hormones in cell metabolism.39 Rerouting DIO3 to the nucleus decreases thyroid-hormone signalling and might function to reduce ischaemia-induced hypoxic brain damage.
The corollary to these findings is that the activity of DIO2 and DIO3 are viewed as a cell-specific, prereceptor mechanism that controls thyroid-hormone signalling, the intensity of which cannot be predicted on the basis of circulating levels of T3.40 For example, cold and/or sympathetic nervous system stimulation of Dio2 expression in rat brown adipose tissue accelerates transcription of T3-responsive genes, such as Ucp1 and Pgc1α. Furthermore, ectopic expression of DIO3 in the human heart and brain during ischaemia or hypoxia42 decreases the amount of T3-dependent transcription in these organs, curbing thyroid-hormone signalling.43,44 Although no humans have been identified with loss-of-function mutations in the iodothyronine deiodinases,45 a few individuals have been identified with defects in selenoprotein synthesis that results in a reduction in DIO2 activity and possible reduction in the activities of DIO1 and DIO3. These patients exhibit increased serum levels of TSH, increased serum levels of T4 and low serum levels of T3, but otherwise have a mild phenotype.46
Effects on brain function
The fundamental impact of thyroid hormones on brain function was established by the striking link between untreated congenital hypothyroidism and developmental retardation of cognitive function.47–49 Myelination, neuronal and/or glial proliferation, differentiation and neuronal migration represent crucial targets of thyroid-hormone-mediated events in the brain.48,50,51 Access of thyroid hormones to the brain is selective, with T4 in plasma having greater ease of access than T3 in plasma because of the types of transporters expressed in the blood–brain barrier.52,53
Uptake of thyroid hormones via the blood–brain barrier and into cells inside the brain parenchyma is mediated by transporters, including monocarboxylate transporter 8 (MCT8) and solute carrier organic anion transporter family member 1C1 (SLCO1C1, also known as OATP1C1).54–56 These transporters are plasma membrane proteins with multiple membrane-spanning domains that have a half-life of several days. Although levels of Mct8 and Slco1c1 mRNA are decreased in a rodent model of nonthyroidal illness,57 currently no direct evidence exists that quick, adaptive changes in the transport of thyroid hormones regulate thyroid hormone availability in the brain. However, the observation that patients with Allan–Herndon–Dudley syndrome express a mutant MCT8 protein explained the molecular background of this rare X-linked disorder, which is characterized by neurological abnormalities (central hypotonia, hearing problems, rotatory nystagmus and spasticity) and developmental delay.58,59
The link between mutations in the genes that encode thyroid hormone transporters and neurological symptoms highlights the critical importance of localized thyroid-hormone signalling in different areas of the brain. In contrast to the majority of tissues, a large portion of T3 in the rodent brain is produced locally via activation of T4. A dual-labelling approach was used to assess this process directly60 and the findings were later confirmed in Dio2 knockout mice that exhibited ~50% reductions in levels of T3 in the brain.61 Thus, local metabolism of thyroid hormones (that is, via the DIO2 and DIO3 pathways) is currently viewed as the major factor that regulates the intensity of thyroid-hormone signalling in the brain. Both enzymes exhibit homeostatic regulation in response to changes in serum levels of thyroid hormones. The activity of DIO2 increases but that of DIO3 decreases in the presence of low concentrations of thyroid hormones (for example, in conditions of hypothyroidism or iodine deficiency), a finding that has been interpreted largely as a mechanism that delays adverse effects of hypothyroidism in the brain.62,63
Neurons are critically important targets of T3; however, these cells lack DIO2 and are unable to generate T3 via this pathway.64–66 Indeed, enrichment of Dio2 expression is detected in glial cells when examining the transcriptome of astrocytes, neurons and oligodendrocytes.67 Furthermore, a >95% drop in Dio2 mRNA and activity occurred in the brain of a mouse model with a transgene conferring glia-specific inactivation of this enzyme.28 Thus, the current paradigm predicts that T3 generated by DIO2 located in astrocytes acts in a paracrine fashion to stimulate TRs in neurons.68–70 This hypothesis has been modelled and validated in vitro by co-culturing human DIO2-expressing glioma cells with human neuronal cells that express DIO3.70 In such a system, T3 generated by glial cells (via DIO2 activity) has a paracrine effect on the co-cultured neurons and activates T3-regulated neuronal genes. Such pathways might also affect thyroid-hormone signalling in vivo. For example, DIO3 is activated by hypoxia inducible factor 1-α under conditions of ischaemia and/or hypoxia.43 Furthermore, DIO2 activity is downregulated and DIO3 expression is activated by the morphogenic hedgehog protein family.71,72
Logically, in responding to ischaemia or hypoxia and other cues known to regulate these enzymes, iodothyronine deiodinases enhance or dampen thyroid-hormone signalling in discrete groups of cells or areas of the brain with clear consequences. For example, in rodent models, Dio2 is involved in the generation of Tsh-releasing hormone (Trh) and/or Tsh feedback (Figure 2). DIO2 is also implicated in the hypothalamic regulation of non-thyroidal illness, seasonal breeding in birds and the onset of hearing in rodents.73–76 Direct evidence suggests that iodothyronine deiodinases have similar roles in humans. For example, in the human fetal hypothalamus, a developmental dependency and coordinated expression of DIO2 and DIO3 occurs in combination with the various thyroid-hormone transporters.77
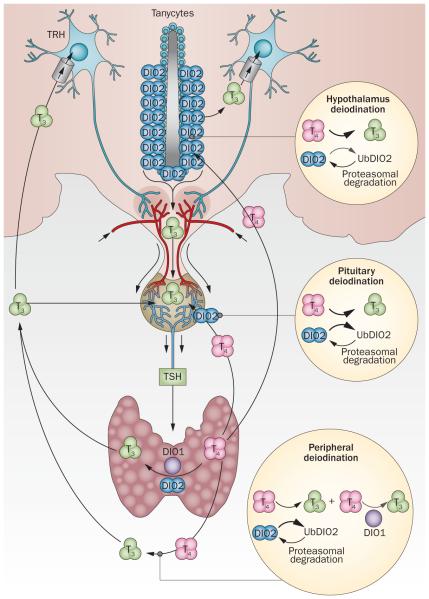
Figure 2.
T4-induced DIO2 ubiquitination in thyroid hormone homeostasis. In response to thyroid hormone signals from the periphery and DIO2-expressing tanycytes, hypophysiotropic TRH-expressing neurons release TRH into the portal blood. TRH is transported to the anterior pituitary gland where TSH is secreted and stimulates the thyroid gland to produce and secrete T4 and T3. Hypothalamic T3 is generated locally by tanycytes and enters the systemic circulation. T3 can also be generated in the periphery via DIO1. In most peripheral tissues, exposure to T4 accelerates inactivation of DIO2 (UbDIO2) and UbDIO2 targeting to the proteasomal system; however, UbDIO2 can be reactivated and rescued fromproteasomal destruction by deubiquitination. Peripheral deiodination is very sensitive to T4-induced DIO2 ubiquitination: a mild increase in the serum T4:T3 ratio favours DIO2 inactivation and decreases fractional T4-to-T3 conversion and peripheral T3 production. However in the hypothalamus, DIO2 is less susceptible to T4-induced ubiquitination than in other tissues. Thus, T4 signalling via DIO2-mediated T3 production is very effective in the hypothalamus, whereas T3 production via DIO2 is easily inhibited in the periphery. Abbreviations: DIO, iodothyronine deiodinase; T2, di-iodothyronine; T3, tri-iodothyronine; T4, tetra-iodothyronine; TRH, TSH-releasing hormone; UbDIO2, ubiquitinated DIO2. Permission obtained from American Society for Clinical Investigation © Werneck de Castro, J. P. et al. J. Clin. Invest. 125, 769–781 (2015).17
In the rodent and avian retina, Dio3 dampens the actions of thyroid hormones, limiting T3 exposure to the cone cells and preserving survival and/or patterning of opsins, which is required for cone function.78 Some evidence supports a role for iodothyronine deiodinase-mediated thyroid-hormone signalling in the human brain. For example, a correlation exists between the levels of thyroid hormones and enzyme activity in different areas of the developing human brain.79 During recovery from intraventricular haemorrhage in preterm infants, which depends on maturation of oligodendrocytes and myelination, DIO2 expression is decreased and DIO3 expression increased, which is indicative of localized hypothyroidism.80 Reversing this effect with levothyroxine treatment promotes neurological recovery in infants and in a model of intraventricular haemorrhage in rabbits and, if confirmed, this approach could improve the neurodevelopmental outcome of preterm infants with intraventricular haemorrhage.80
What changes in hypothyroidism?
The amount of thyroid hormone entering a cell is equivalent to the amount exiting that cell, characterizing a state of equilibrium. Studies in animal models and human cells grown in culture suggest that the net flow of T4 and T3 depends on the type of iodothyronine deiodinase expressed in each given cell type (Figure 1). The expression of DIO2 creates an inward net flow of T4 and an outward net flow of T3.40 Expression of DIO3 creates inward net flows of both T4 and T3, whereas the net flow is neutral if no iodothyronine deiodinase is expressed.40
Iodothyronine deiodinase activity also affects circulating levels of T4 and T3. In humans, ~70% of the circulating T3 is produced via the extrathyroidal DIO2 pathway, whereas ~15% originates from the DIO1 pathway. Given that DIO1 is positively regulated by T3,81 whereas the opposite is seen for DIO2, the contribution of DIO1 to the circulating T3 pool is increased in patients with hyperthyroidism. Notably, DIO1 activity is inhibited by propylthiouracil, glucocorticoids and β blockers, which explains at least part of the clinical efficacy of these drugs.40
Most circulating T3 is metabolized via the DIO3 pathway.82 This pathway acquires particular clinical relevance during pregnancy because DIO3 is highly expressed in the human placenta,83 and, therefore, increases the daily requirements for thyroid hormone for patients on thyroid hormone replacement therapy.84 Increased expression of DIO3 is also observed in some disease states, including in the liver, lungs, heart and brain of patients experiencing ischaemia or hypoxia.42 In rare cases, DIO3 is expressed in infantile haemangioma to such an extent that it inactivates T3 at a faster rate than the hormone can be produced (consumptive hypothyroidism).85 Similarly, treatment of patients who have cancer with the tyrosine kinase inhibitors imatinib and sunitinib is associated with the development of hypothyroidism, which seems to be the result of marked overexpression of DIO3 within the tumour cells.86
Hypothyroidism is an important example of the mass effect of the iodothyronine deiodinases changing their levels of activity in a coordinated fashion. As a result of low thyroid hormone levels, the activity of DIO2 is accelerated in almost all tissues, increasing the whole-body fractional conversion of T4 to T3 that helps preserve serum levels of T3 (the opposite is observed during hyperthyroidism).87 In addition, DIO3 is a T3-responsive gene and thus T3 clearance is reduced in hypothyroidism. These coordinated and reciprocal enzyme responses explain why measuring serum concentrations of T3 is of little diagnostic value in patients with hypothyroidism.
The DIO2-adaptive response to hypothyroidism is possible because of the unique sensitivity of this enzyme to T4, its natural substrate. DIO2 has a short half-life (~60 min) that becomes even shorter (20 min) as a result of interacting with T4 and/or its catalytic activity.88,89 In other words, by converting T4 to T3, DIO2 is inactivated and degraded. This mechanism is explained by an 18-amino acid instability loop unique to the DIO2 protein that is located adjacent to its globular catalytic domain.71 This loop mediates binding to the hedgehog-inducible protein WD repeat and SOCS box-containing protein 1 (WSB-1), a ubiquitin ligase adaptor that mediates DIO2 ubiquitination and targeting for proteasomal degradation.90 Truncation analyses identified a core of six amino acids within the loop as the minimal requirements critical for recognition of DIO2 by WSB-1.91 The loop explains the short half-life of DIO2, a characteristic that can be transferred between proteins if the loop is fused to an otherwise stable protein.33
As with the other iodothyronine deiodinases, DIO2 exists as a dimer that is maintained by interacting surfaces within its transmembrane and globular cytosolic domains.92 Upon binding T4, DIO2 is ubiquitinated with ubiquitin chains formed at the Lys48 position,93 which in turn inactivates the enzyme by interfering with globular interacting surfaces critical for dimerization and catalytic activity.90 This inactive state can be transient or permanent, depending on whether ubiquitinated DIO2 is reactivated by DIO2-interacting deubiquitinases (such as ubiquitin specific peptidase 33),94 or retrotranslocated to the cytoplasm via the p97–ATPase complex and delivered to the proteasomes.93 The continuous association of DIO2 with this regulatory protein complex supports rapid cycles of deiodination, conjugation to ubiquitin and enzyme reactivation by deubiquitination, which enables tight control of thyroid-hormone action (Figure 2).
Treatment of hypothyroidism
Limitations of levothyroxine monotherapy
Unique aspects of hypothalamic DIO2 define limitations of levothyroxine replacement therapy and the use of TSH as a therapeutic goal. Circulating T4 and T3 exert negative feedback on the secretion of TRH and TSH.76 In rodents, Tsh-secreting cells co-express Dio2;95 in the rodent hypothalamus, Dio2 is expressed in specialized glial cells known as tanycytes (Figure 2). These cells are located in the mediobasal hypothalamus, as well as on the floor and infralateral wall of the third ventricle. Tanycytes are distributed from the end of the optic chiasm, along the mammillary recess, and their processes also reach the median eminence outside the blood–brain barrier.64,65,76 The presence of DIO2 in both locations is critical for T4-mediated negative feedback.28,96 In the pituitary gland, T4 must be converted to T3 to suppress secretion of TSH. In the hypothalamus, T3 generation via DIO2 in tanycytes is likely to negatively affect TRH expression in the paraventricular nucleus.97 However, the full extent of the function of DIO2 at both sites is only just starting to be appreciated.17 Given that DIO2 activity is increased in most tissues during hypothyroidism and decreased in hyperthyroidism (via ubiquitination), its presence in the mediobasal hypothalamus and thyrotropic cells would seem to be counterproductive to the feedback mechanism. Thus, for many years it remained unclear how fluctuations in plasma levels of T4 could be faithfully transduced in the nuclei of thyrotropic cells and hypophysiotropic TRH neurons in the hypothalamic paraventricular nucleus to ultimately regulate serum concentrations of TSH.95
The key new element in this feedback mechanism is the observation that rat hypothalamic Dio2 is stable and largely refractory to the levels of thyroid hormones.17 In contrast to the rest of the body, DIO2-mediated T4-to-T3 conversion in the hypothalamus is not accelerated during hypothyroidism nor is it diminished by hyperthyroidism or administration of thyroid hormones. Indeed, a sensitivity gradient exists in the DIO2 response to hypothyroidism and thyroid hormones between the hypothalamus and the rest of the brain and body,17 which is similar to previous observations in rodent thyrotropic cells.95 The corollary of these experiments is that the secretion of Trh and Tsh in rodents is controlled by a steady process of Dio2-mediated T4-to-T3 conversion, thus transducing minor changes in serum levels of T4 (Figure 2). By contrast, the rate of Dio2-mediated T4-to-T3 conversion in the rest of the body progressively decreases with administration of thyroid hormones because of Dio2 ubiquitination, so that peripheral T3 production in levothyroxine-treated hypothyroid rats is progressively decreased and self-limiting. As a result, the dose of levothyroxine required to normalize serum concentrations of TSH is lower than the dose that normalizes serum levels of T3. Given that the rat model reproduces the findings observed among patients treated with levothyroxine, similar pathways in humans are expected to provide the mechanistic basis for the observation of an increased ratio of serum T4 to T3 in the setting of normalized serum levels of TSH that is frequently observed among patients treated with levothyroxine.12
Differences in hypothalamic DIO2 susceptibility to ubiquitination explain localized sensitivity to levothyroxine.17 Both in vivo studies in mice harbouring astrocyte-specific inactivation of Wsb1 and in vitro analysis of DIO2 ubiquitination induced by different tissue extracts indicated that DIO2 ubiquitination in the hypothalamus is lower or that deubiquitination is faster than in other tissues.17 As a result, in contrast to other DIO2-expressing tissues, the hypothalamus is wired to have increased sensitivity to T4.
Hypothalamic DIO2 is also sensitive to other stimuli that are involved in the hypothalamic–pituitary–thyroid axis, including nutritional and inflammatory signals.76 The regulation of DIO2 activity in tanycytes, therefore, also integrates other signals that override the input provided by circulating levels of thyroid hormone. For example, in a rodent model of lipopolysaccharide-induced nonthyroidal illness, Dio2 is upregulated in tanycytes, which results in increased local thyroid-hormone signalling that in turn suppresses TRH expression despite falling serum levels of thyroid hormone.98,99 Notably, lipopolysaccharide-mediated downregulation of Trh does not occur in mice with global inactivation of Dio2, which indicates that this pathway is required for this process.70 Studies in transgenic mice suggest that mice without appreciable Dio2 expression in astrocytes, but with an intact tanycyte Dio2 pathway, can efficiently regulate their hypothalamic–pituitary–thyroid axis.28
Normalization of T3 levels in hypothyroidism
Many patients receiving levothyroxine do not achieve serum levels of T3 within the normal range and have raised serum levels of T4, which results in a high serum T4:T3 ratio.12,100,101 Although this phenomenon has been recognized for many decades, clinicians generally adopted the hypothesis that iodothyronine deiodinases would appropriately regulate the pool of available T3 at the cellular and tissue level, thus leaving patients treated with levothyroxine euthyroid. However, some investigators have questioned whether a low serum level of T3 and/or a high T4:T3 ratio could have clinical implications. Specifically, whether this situation causes the residual symptoms experienced by a minority of patients treated with levothyroxine.9,102
The clinical data remain contentious.15,18,19 However, studies in rats indicate that levothyroxine administration alone does not normalize serum Tsh, serum T3 and tissue T3 levels at the same time,18 and that a serum Tsh level within the normal range coexists with reduced serum and tissue T3 levels. These issues have been confirmed in an animal study in which thyroidectomized rats receiving levothyroxine monotherapy exhibited low levels of serum T3 and a high T4:T3 ratio.17 The novelty of this study was not only that these abnormalities in systemic parameters were confirmed but also that tissue-specific markers of hypothyroidism were evaluated. Mitochondrial content and α-glycerophosphate dehydrogenase activity—both known markers of T3-responsiveness in the liver and skeletal muscle—were normalized in rats receiving levothyroxine plus liothyronine combination therapy but not in those treated with levothyroxine alone.17 In addition, serum levels of cholesterol were normalized in rats receiving combination therapy but not in those receiving levothyroxine monotherapy.
Therefore, in the rodent model, levothyroxine replacement therapy that results in a high ratio of T4 to T3 and low serum levels of T3 seem to exert consequences on markers of hypothyroidism at the tissue level (liver and skeletal muscle). The application of these findings to human studies will be important to determine whether patients treated with levothyroxine experience tissue-specific hypothyroidism and, if so, whether this effect can be reversed with combination therapy.
Thyroid-hormone homeostasis in the brain
The plasma contributes approximately half of the T3 present in the rodent brain, with the remainder produced locally via deiodination of T4;61 therefore, a drop in serum levels of T4 or T3 could negatively affect thyroid-hormone signalling in the brain. However, this effect is largely minimized by the homeostatic actions of the iodothyronine deiodinases.63 Accordingly, normalization of T3 levels in the cerebral cortex, but not in the cerebellum, in levothyroxine-treated thyroidectomized rats is achieved when circulating levels of T4 and T3 are half that of euthyroid control animals that did not undergo thyroidectomy.18 In addition, levels of T3 in the cerebral cortex remain within the normal range, despite an up to 20-fold increase in the levothyroxine infusion doses.
These landmark studies provide the rationale for very tight control of thyroid hormone actions in the brain. These findings suggest that thyroid-hormone signalling is preserved in the brains of patients with mild hypothyroidism. However, the different behaviour of rat cerebral cortex and cerebellum confirm that thyroid-hormone signalling in the brain is highly compartmentalized (that is, not all brain areas behave in the same way). To address this issue, the action of thyroid hormone was studied using 16 genetic markers (such as ENPP2) of T3-responsiveness in the cerebral cortex, cerebellum and hippocampus of thyroidectomized rats treated with either levothyroxine monotherapy or levothyroxine plus liothyronine combination therapy at doses that normalize serum levels of Tsh.17 Expression of all genetic markers returned to normal levels in thyroidectomized rats receiving combination therapy, whereas 10 markers failed to be normalized in those receiving monotherapy, which is indicative of cerebral hypothyroidism.17 The animals receiving monotherapy had reduced serum levels of T3, which might explain some of these observations. An additional explanation is that the reduction of Dio2 activity in the brain observed in levothyroxine-treated animals (caused by the raised serum T4:T3 ratio) could lead to decreased T3 production via Dio2 and add to the localized hypothyroidism.17
These findings challenge the idea that the impact of reduced DIO2 activity on T3 production following exposure to T4 is compensated for by increased substrate availability, thus preserving or even increasing T3 production. Although logical, this rationale might not apply to all DIO2-expressing tissue or cell types given that in a cell system in which conversion of T4 to T3 via DIO2 was monitored while cells were exposed to progressively higher T4 levels, T3 production was lower than the T4 concentration in the medium only to a limited extent.95 This change was followed by an inflection point at which further increases in T4 concentration reduced DIO2 activity, and T3 production fell abruptly; the level of T4 at the inflection point depends on the cell type, presumably because of differences in DIO2 ubiquitination.95 This finding is supported by the observations that Dio2 activity in mice with brain-specific inactivation of Wsb-1 respond differently to T4 administration across different brain areas.17 For example, Wsb-1 mediates Dio2 inactivation in response to levothyroxine administration in brain areas such as the cerebral cortex and hippocampus, whereas in the cerebellum, Wsb-1 does not mediate loss of Dio2 activity. Furthermore, levothyroxine-induced Dio2 inactivation is minimal (almost nonexistent), in the hypothalamus.17
Mood disorders and cognition
To what extent do the mechanisms mediated by iodothyronine deiodinase that regulate thyroid-hormone signalling underpin clinical phenotypes observed in patients with hypothyroidism? These patients have variable degrees of cognitive dysfunction, lethargy, poor motor coordination, memory impairment, depression and mood disorders. In addition, the efficacy of antidepressant agents among euthyroid individuals is potentiated when associated with the administration of thyroid hormone, either levothyroxine or liothyronine.103,104 In some clinical studies, but not all,105 a residual cognitive dysfunction exists in patients with hypothyroidism who are treated with levothyroxine.9,53,106,107 Whether these residual symptoms are a consequence of low serum levels of T3, or a high T4 to T3 ratio,108,109 remains to be determined. In addition, whether combination therapy with levothyroxine and liothyronine can restore cognitive function is an interesting hypothesis that needs to be tested.
Some evidence suggests that thyroid hormones act in the brain via serotonergic pathways, a system involved in the pathogenesis of affective disorders and response to psychotropic agents.110 Arising from discrete brain-stem nuclei, serotonin circuits project to cortical and subcortical brain areas, including the prefrontal cortex, hippocampus and amygdala, which explains their influence on behaviours such as mood, sleep and appetite.111 The use of MRI spectroscopy and PET has confirmed that thyroid hormones act in the human limbic system, with substantial differences observed in the posterior cingulate cortex and the inferior parietal lobe of hypothyroid versus hyperthyroid individuals.112,113
Considering that iodothyronine deiodinases modify thyroid-hormone signalling in the rodent brain,114 it seems logical to assume that the activity of these enzymes might affect mood and/or have a role in the variability of the response of patients with depression to T3. In animal models, Dio2 activity in the brain is increased by antidepressants of various classes, including selective serotonin reuptake inhibitors.115–117 In addition, hippocampal expression of Dio2 and Dio3 is increased in a mouse model that is resistant to stress-induced depression and after antidepressant treatment in mice.108 However, a mouse with global inactivation of Dio2 exhibits only a mild motor phenotype and no behavioural phenotype.61,118 By contrast, a mouse model with global inactivation of Dio3 exhibits brain hyperthyroidism, aggressive behaviour and indifference towards pups.119 Furthermore, a negative correlation exists between Dio3 expression and T3 levels in the frontal cortex and hippocampus of a mouse model of depressive behaviour and cognitive deficits, which is partially restored by administration of levothyroxine.120–122
The available clinical data are inconsistent for DIO2 and DIO3 but strong for DIO1, which is expressed at low levels in the human brain.24,79 A study of 1,447 individuals enrolled from the general population indicated that a polymorphism in DIO1, called C785T, is associated with the lifetime risk of depression in white female individuals in high-risk cohorts123 and with the antidepressant efficacy of T3 in a trial of the selective serotonin reuptake inhibitor sertraline.124
Genetic risk and brain disease
A prevalent single nucleotide polymorphism in the DIO2 gene (which results in a single amino acid substitution of Thr for Ala at position 92 in DIO2, thus called Thr92AlaD2) has been identified,125 with estimates suggesting that ~12–36% of individuals in the general population are homozygous. This variant has been associated with metabolic derangements, including insulin resistance125,126 and type 2 diabetes mellitus.127 Furthermore, this polymorphism has been implicated in mental and psychological disorders,45 such as mental retardation,128 bipolar disorder129 and low IQ.130 Conversely, Thr92AlaD2 might confer protection from recurrent depression.131 The clinical relevance of Thr92AlaD2 was strengthened in a large clinical trial, in which the substitution was correlated with reduced well-being and a preference for levothyroxine plus liothyronine combination therapy versus levothyroxine monotherapy among carriers with hypothyroidism.13
These findings have stimulated much intrigue in the field with most initial hypotheses focusing on a possible defect in thyroid-hormone signalling. However, the single amino acid substitution associated with Thr92AlaD2 does not alter the enzyme kinetics of DIO2 when transiently expressed in cells132,133 and only indirect evidence suggests that the conversion of T4 to T3 via Thr92AlaD2 could be reduced in patients.134,135 Perhaps this observation is not unexpected given that the mutation lies not within the catalytic site but rather within the 18-amino acid loop that controls susceptibility to T4-induced ubiquitination of DIO2.31 The putative functional abnormality associated with expression of the variant protein was defined in studies performed in a human embryonic kidney cell line that had been engineered to stably express this mutation. The mutated protein has a longer half-life than the native form and is ectopically located in the Golgi apparatus.35 This aberrant subcellular localization was associated with abnormal Golgi structure, such that circular (rather than linear or ribbon-like) stacks of Golgi membrane were observed. These cellular abnormalities resulted in alterations at the transcriptional level that were independent of DIO2-mediated T3 production given that the cell line studied does not express TRs136 and that the observed gene expression pattern lacked typical indicators of T3-responsiveness.114 Remarkably, when human cerebral cortex samples from patients harbouring the polymorphism were studied, overlap in the expression of 81 genes was found, which defines a molecular finger-print associated with the Thr92AlaD2 variant across these human cell and brain models.35
In addition, robust analysis of microarray data derived from human brain samples demonstrated a gene expression profile reminiscent of that of neurological diseases, particularly the gene expression profile associated with Huntington disease.35 This finding implies that the Thr92AlaD2 polymorphism is a novel risk factor for neurodegenerative disease or impaired cognition, which might explain the neuropsychological impairment of some patients; however, further studies are needed before definite conclusions can be drawn.35 In particular, elucidating whether the high T4:T3 ratio observed in patients with hypothyroidism who are treated with levothyroxine12,100 might perturb the cellular abnormalities associated with expression of the variant protein will be important. This relationship could explain the improved well-being and preference for combination therapy recorded in a subgroup of patients, as no evidence of reduced thyroid-hormone signalling in the samples was found (T3-independent).13
These findings represent a step towards determining the relationship between the cellular abnormalities associated with variant protein expression and the associated clinical phenotype. Future studies are needed to fully characterize the molecular basis for the clinical observations. Nevertheless, clinical trials among this subgroup of patients could be warranted to determine whether patients with hypothyroidism who carry the polymorphism would benefit from alternative therapeutic strategies. If it can be rigorously demonstrated that such patients derive benefit from combination therapy, then genotyping for Thr92AlaD2 could become a routine component of hypothyroidism management, thus bringing the concept of personalized medicine to the field.
Conclusions
Major advances have been made in the treatment of hypothyroidism over the past century; however, refined therapeutic regimens might still be needed to ensure that all patients can become asymptomatic and clinically euthyroid. Once heralded as the ultimate regulators of thyroid-hormone availability and the key to levothyroxine treatment efficacy, iodothyronine deiodinases might actually underpin the inability to normalize serum levels of TSH and T3 in patients receiving levothyroxine monotherapy and the insufficient symptomatic response experienced by an appreciable proportion of patients with hypothyroidism. Future studies exploring the role of the iodothyronine deiodinases in hypothyroidism hold real promise for determining therapeutic regimens that can normalize systemic and tissue-level parameters for all patients with hypothyroidism, including new approaches that might even be genotype-directed.
Key points.
Levothyroxine monotherapy at doses that normalize serum levels of TSH does not universally restore parameters of thyroid hormone levels for patients with hypothyroidism
The iodothyronine deiodinases provide a cell-specific, prereceptor mechanism that controls thyroid-hormone signalling
Localized thyroid-hormone signalling has a critical role in different areas of the brain, as mediated by thyroid hormone transporters and the iodothyronine deiodinases
DIO2 ubiquitination induced by tetraiodothyronine (T4) normally decreases tri-iodothyronine (T3) production, but not in the hypothalamus
The levothyroxine dose that normalizes serum levels of TSH in an animal model is lower than the dose that normalizes serum levels of T3, which explains the increased serum T4 to T3 ratio observed in patients treated with levothyroxine
>If patients carrying the Thr92AlaD2 polymorphism derive benefit from combination therapy with levothyroxine and liothyronine, then genotyping for this single nucleotide polymorphism might become a component of the management of hypothyroidism
Acknowledgements
B.G. is supported by the Hungarian Brain Research Program and Hungarian Scientific Research Fund (OTKA 109415). M.O.R. is supported by CAPES and FAPESP. A.C.B. is supported by the NIDDK.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
All authors contributed to researching the data for the article and the writing the article. B.G., E.A.M. and A.C.B. provided a substantial contribution to discussions of the content and reviewed and/or edited the manuscript before submission.
Contributor Information
Balázs Gereben, Department of Endocrine Neurobiology, Institute of Experimental Medicine, Hungarian Academy of Sciences, Szigony Street 43, Budapest H-1083, Hungary.
Elizabeth A. McAninch, Division of Endocrinology and Metabolism, Rush University Medical Center, 212 Cohn Building, 1735 West Harrison Street, Chicago, IL 60612, USA
Miriam O. Ribeiro, Developmental Disorders Program, Center for Biological and Health Science, Mackenzie Presbyterian University, Rua da Consolação 930, Building 16, São Paulo, SP 01302, Brazil
Antonio C. Bianco, Division of Endocrinology and Metabolism, Rush University Medical Center, 212 Cohn Building, 1735 West Harrison Street, Chicago, IL 60612, USA
References
- 1.Aoki Y, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002) Thyroid. 2007;17:1211–1223. doi: 10.1089/thy.2006.0235. [DOI] [PubMed] [Google Scholar]
- 2.Vanderpump MP. The epidemiology of thyroid disease. Br. Med. Bull. 2011;99:39–51. doi: 10.1093/bmb/ldr030. [DOI] [PubMed] [Google Scholar]
- 3.Lindholm J, Laurberg P. Hypothyroidism and thyroid substitution: historical aspects. J. Thyroid Res. 2011;2011:809341. doi: 10.4061/2011/809341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braverman LE, Ingbar SH, Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic subjects. J. Clin. Invest. 1970;49:855–864. doi: 10.1172/JCI106304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsen PR, Ingbar S. In: Textbook of Endocrinology. Wilson JD, et al., editors. W. B. Saunders, Co.; 1992. pp. 357–487. [Google Scholar]
- 6.Garber JR, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr. Pract. 2012;18:988–1028. doi: 10.4158/EP12280.GL. [DOI] [PubMed] [Google Scholar]
- 7.Taylor S, Kapur M, Adie R. Combined thyroxine and triiodothyronine for thyroid replacement therapy. Br. Med. J. 1970;2:270–271. doi: 10.1136/bmj.2.5704.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts ND. Psychological problems in thyroid disease. British Thyroid Foundation Newsletter. 1996;18:3. [Google Scholar]
- 9.Saravanan P, et al. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin. Endocrinol. 2002;57:577–585. doi: 10.1046/j.1365-2265.2002.01654.x. [DOI] [PubMed] [Google Scholar]
- 10.Gorman CA, Jiang NS, Ellefson RD, Elveback LR. Comparative effectiveness of dextrothyroxine and levothyroxine in correcting hypothyroidism and lowering blood lipid levels in hypothyroid patients. J. Clin. Endocrinol. Metab. 1979;49:1–7. doi: 10.1210/jcem-49-1-1. [DOI] [PubMed] [Google Scholar]
- 11.Franklyn JA, et al. Thyroxine replacement therapy and circulating lipid concentrations. Clin. Endocrinol. 1993;38:453–459. doi: 10.1111/j.1365-2265.1993.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 12.Gullo D, et al. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS ONE. 2011;6:e22552. doi: 10.1371/journal.pone.0022552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panicker V, et al. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J. Clin. Endocrinol. Metab. 2009;94:1623–1629. doi: 10.1210/jc.2008-1301. [DOI] [PubMed] [Google Scholar]
- 14.Bunevicius R, Kazanavicius G, Zalinkevicius R, Prange AJ., Jr. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N. Engl. J. Med. 1999;340:424–429. doi: 10.1056/NEJM199902113400603. [DOI] [PubMed] [Google Scholar]
- 15.Escobar-Morreale HF, et al. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann. Intern. Med. 2005;142:412–424. doi: 10.7326/0003-4819-142-6-200503150-00007. [DOI] [PubMed] [Google Scholar]
- 16.Jonklaas J, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Werneck de Castro JP, et al. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J. Clin. Invest. 2015;125:769–781. doi: 10.1172/JCI77588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escobar-Morreale HF, Obregon MJ, Escobar del Rey F, Morreale de Escobar G. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J. Clin. Invest. 1995;96:2828–2838. doi: 10.1172/JCI118353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Escobar-Morreale HF, Rey F, Obregon MJ, Escobar GM. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology. 1996;137:2490–2502. doi: 10.1210/endo.137.6.8641203. [DOI] [PubMed] [Google Scholar]
- 20.Escobar-Morreale HF, Obregon MJ, Hernandez A, Escobar del Rey F, Morreale de Escobar G. Regulation of iodothyronine deiodinase activity as studied in thyroidectomized rats infused with thyroxine or triiodothyronine. Endocrinology. 1997;138:2559–2568. doi: 10.1210/endo.138.6.5212. [DOI] [PubMed] [Google Scholar]
- 21.Bianco AC, et al. American Thyroid Association guide to investigating thyroid hormone economy and action in rodent and cell models. Thyroid. 2014;24:88–168. doi: 10.1089/thy.2013.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gereben B, Salvatore D, Harney JW, Tu HM, Larsen PR. The human, but not rat, Dio2 gene is stimulated by thyroid transcription factor-1 (TTF-1) Mol. Endocrinol. 2001;15:112–124. doi: 10.1210/mend.15.1.0579. [DOI] [PubMed] [Google Scholar]
- 23.Salvatore D, Tu H, Harney JW, Larsen PR. Type 2 iodothyronine deiodinase is highly expressed in human thyroid. J. Clin. Invest. 1996;98:962–968. doi: 10.1172/JCI118880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campos-Barros A, et al. Phenolic and tyrosyl ring iodothyronine deiodination and thyroid hormone concentrations in the human central nervous system. J. Clin. Endocrinol. Metab. 1996;81:2179–2185. doi: 10.1210/jcem.81.6.8964848. [DOI] [PubMed] [Google Scholar]
- 25.Visser TJ, Leonard JL, Kaplan MM, Larsen PR. Kinetic evidence suggesting two mechanisms for iodothyronine 5'-deiodination in rat cerebral cortex. Proc. Natl Acad. Sci. USA. 1982;79:5080–5084. doi: 10.1073/pnas.79.16.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christoffolete MA, et al. Mice with impaired extrathyroidal thyroxine to 3,5,3'-triiodothyronine conversion maintain normal serum 3,5,3'-triiodothyronine concentrations. Endocrinology. 2007;148:954–960. doi: 10.1210/en.2006-1042. [DOI] [PubMed] [Google Scholar]
- 27.Fonseca TL, et al. Tissue-specific inactivation of type 2 deiodinase reveals multilevel control of fatty acid oxidation by thyroid hormone in the mouse. Diabetes. 2014;63:1594–1604. doi: 10.2337/db13-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonseca TL, et al. Coordination of hypothalamic and pituitary T3 production regulates TSH expression. J. Clin. Invest. 2013;123:1492–1500. doi: 10.1172/JCI61231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brent GA. Mechanisms of thyroid hormone action. J. Clin. Invest. 2012;122:3035–3043. doi: 10.1172/JCI60047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heuer H, Visser TJ. The pathophysiological consequences of thyroid hormone transporter deficiencies: Insights from mouse models. Biochim. Biophys. Acta. 2013;1830:3974–3978. doi: 10.1016/j.bbagen.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Callebaut I, et al. The iodothyronine selenodeiodinases are thioredoxin-fold family proteins containing a glycoside hydrolase clan GH-A-like structure. J. Biol. Chem. 2003;278:36887–36896. doi: 10.1074/jbc.M305725200. [DOI] [PubMed] [Google Scholar]
- 32.Schweizer U, Schlicker C, Braun D, Kohrle J, Steegborn C. Crystal structure of mammalian selenocysteine-dependent iodothyronine deiodinase suggests a peroxiredoxin-like catalytic mechanism. Proc. Natl Acad. Sci. USA. 2014;111:10526–10531. doi: 10.1073/pnas.1323873111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeold A, et al. Metabolic instability of type 2 deiodinase is transferable to stable proteins independently of subcellular localization. J. Biol. Chem. 2006;281:31538–31543. doi: 10.1074/jbc.M604728200. [DOI] [PubMed] [Google Scholar]
- 34.Baqui MM, Gereben B, Harney JW, Larsen PR, Bianco AC. Distinct subcellular localization of transiently expressed types 1 and 2 iodothyronine deiodinases as determined by immunofluorescence confocal microscopy. Endocrinology. 2000;141:4309–4312. doi: 10.1210/endo.141.11.7872. [DOI] [PubMed] [Google Scholar]
- 35.McAninch EA, et al. Prevalent polymorphism in thyroid hormone-activating enzyme leaves a genetic fingerprint that underlies associated clinical syndromes. J. Clin. Endocrinol. Metab. 2015;100:920–933. doi: 10.1210/jc.2014-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider MJ, et al. Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology. 2006;147:580–589. doi: 10.1210/en.2005-0739. [DOI] [PubMed] [Google Scholar]
- 37.Baqui M, et al. Human type 3 iodothyronine selenodeiodinase is located in the plasma membrane and undergoes rapid internalization to endosomes. J. Biol. Chem. 2003;278:1206–1211. doi: 10.1074/jbc.M210266200. [DOI] [PubMed] [Google Scholar]
- 38.Kallo I, et al. A novel pathway regulates thyroid hormone availability in rat and human hypothalamic neurosecretory neurons. PLoS ONE. 2012;7:e37860. doi: 10.1371/journal.pone.0037860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jo S, et al. Neuronal hypoxia induces hsp40-mediated nuclear import of type 3 deiodinase as an adaptive mechanism to reduce cellular metabolism. J. Neurosci. 2012;32:8491–8500. doi: 10.1523/JNEUROSCI.6514-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gereben B, et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr. Rev. 2008;29:898–938. doi: 10.1210/er.2008-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bianco AC, McAninch EA. The role of thyroid hormone and brown adipose tissue in energy homoeostasis. Lancet Diabetes Endocrinol. 2013;1:250–258. doi: 10.1016/S2213-8587(13)70069-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peeters RP, et al. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J. Clin. Endocrinol. Metab. 2003;88:3202–3211. doi: 10.1210/jc.2002-022013. [DOI] [PubMed] [Google Scholar]
- 43.Simonides WS, et al. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J. Clin. Invest. 2008;118:975–983. doi: 10.1172/JCI32824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olivares EL, et al. Thyroid function disturbance and type 3 iodothyronine deiodinase induction after myocardial infarction in rats a time course study. Endocrinology. 2007;148:4786–4792. doi: 10.1210/en.2007-0043. [DOI] [PubMed] [Google Scholar]
- 45.Bianco AC, Casula S. Thyroid hormone replacement therapy: three ‘simple’ questions, complex answers. Eur. Thyroid J. 2012;1:88–98. doi: 10.1159/000339447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dumitrescu AM, et al. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat. Genet. 2005;37:1247–1252. doi: 10.1038/ng1654. [DOI] [PubMed] [Google Scholar]
- 47.Ord WM. Report of the committee of the Clinical Society of London nominated December 14, 1883, to investigate the subject of myxoedema. Trans. Clin. Soc. Lond. 1888;21(Suppl.):1–215. [Google Scholar]
- 48.Oppenheimer JH, Schwartz HL. Molecular basis of thyroid hormone-dependent brain development. Endocr. Rev. 1997;18:462–475. doi: 10.1210/edrv.18.4.0309. [DOI] [PubMed] [Google Scholar]
- 49.Obregon MJ, Escobar del Rey F, Morreale de Escobar G. The effects of iodine deficiency on thyroid hormone deiodination. Thyroid. 2005;15:917–929. doi: 10.1089/thy.2005.15.917. [DOI] [PubMed] [Google Scholar]
- 50.Desouza LA, et al. Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol. Cell. Neurosci. 2005;29:414–426. doi: 10.1016/j.mcn.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Bernal J. Thyroid hormone receptors in brain development and function. Nat. Clin. Pract. Endocrinol. Metab. 2007;3:249–259. doi: 10.1038/ncpendmet0424. [DOI] [PubMed] [Google Scholar]
- 52.Morte B, Bernal J. Thyroid hormone action: astrocyte-neuron communication. Front. Endocrinol. 2014;5:82. doi: 10.3389/fendo.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenberg JH, et al. Imaging triiodothyronine binding kinetics in rat brain: a model for studies in human subjects. Synapse. 2006;60:212–222. doi: 10.1002/syn.20293. [DOI] [PubMed] [Google Scholar]
- 54.Visser TJ. Thyroid hormone transporters. Horm. Res. 2007;68(Suppl. 5):28–30. doi: 10.1159/000110469. [DOI] [PubMed] [Google Scholar]
- 55.Ceballos A, et al. Importance of monocarboxylate transporter 8 for the blood-brain barrier-dependent availability of 3,5,3'-triiodo-L-thyronine. Endocrinology. 2009;150:2491–2496. doi: 10.1210/en.2008-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liao XH, et al. Distinct roles of deiodinases on the phenotype of Mct8 defect: a comparison of eight different mouse genotypes. Endocrinology. 2011;152:1180–1191. doi: 10.1210/en.2010-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wittmann G, et al. Parallel regulation of thyroid hormone transporters OATP1c1 and MCT8 during and after endotoxemia at the blood-brain barrier of male rodents. Endocrinology. 2015;156:1552–1564. doi: 10.1210/en.2014-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friesema EC, et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364:1435–1437. doi: 10.1016/S0140-6736(04)17226-7. [DOI] [PubMed] [Google Scholar]
- 59.Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am. J. Hum. Genet. 2004;74:168–175. doi: 10.1086/380999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crantz FR, Silva JE, Larsen PR. Analysis of the sources and quantity of 3,5,3'-triiodothyronine specifically bound to nuclear receptors in rat cerebral cortex and cerebellum. Endocrinology. 1982;110:367–375. doi: 10.1210/endo-110-2-367. [DOI] [PubMed] [Google Scholar]
- 61.Galton VA, et al. Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient rodent brain during development. Endocrinology. 2007;148:3080–3088. doi: 10.1210/en.2006-1727. [DOI] [PubMed] [Google Scholar]
- 62.Silva JE, Matthews PS. Production rates and turnover of triiodothyronine in rat-developing cerebral cortex and cerebellum: responses to hypothyroidism. J. Clin. Invest. 1984;74:1035–1049. doi: 10.1172/JCI111471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peeters R, et al. Regional physiological adaptation of the central nervous system deiodinases to iodine deficiency. Am. J. Physiol. Endocrinol. Metab. 2001;281:E54–E61. doi: 10.1152/ajpendo.2001.281.1.E54. [DOI] [PubMed] [Google Scholar]
- 64.Guadano-Ferraz A, Obregon MJ, St Germain DL, Bernal J. The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proc. Natl Acad. Sci. USA. 1997;94:10391–10396. doi: 10.1073/pnas.94.19.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tu HM, et al. Regional distribution of type 2 thyroxine deiodinase messenger ribonucleic acid in rat hypothalamus and pituitary and its regulation by thyroid hormone. Endocrinology. 1997;138:3359–3368. doi: 10.1210/endo.138.8.5318. [DOI] [PubMed] [Google Scholar]
- 66.Mohacsik P, Zeold A, Bianco AC, Gereben B. Thyroid hormone and the neuroglia: both source and target. J. Thyroid Res. 2011;2011:215718. doi: 10.4061/2011/215718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cahoy JD, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bernal J, Guadano-Ferraz A, Morte B. Perspectives in the study of thyroid hormone action on brain development and function. Thyroid. 2003;13:1005–1012. doi: 10.1089/105072503770867174. [DOI] [PubMed] [Google Scholar]
- 69.Visser WE, Friesema EC, Jansen J, Visser TJ. Thyroid hormone transport by monocarboxylate transporters. Best Pract. Res. Clin. Endocrinol. Metab. 2007;21:223–236. doi: 10.1016/j.beem.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Freitas BC, et al. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J. Clin. Invest. 2010;120:2206–2217. doi: 10.1172/JCI41977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dentice M, et al. The Hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the developing growth plate. Nat. Cell Biol. 2005;7:698–705. doi: 10.1038/ncb1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dentice M, et al. Sonic hedgehog-induced type 3 deiodinase blocks thyroid hormone action enhancing proliferation of normal and malignant keratinocytes. Proc. Natl Acad. Sci. USA. 2007;104:14466–14471. doi: 10.1073/pnas.0706754104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fliers E, Alkemade A, Wiersinga WM, Swaab DF. Hypothalamic thyroid hormone feedback in health and disease. Prog. Brain Res. 2006;153:189–207. doi: 10.1016/S0079-6123(06)53011-0. [DOI] [PubMed] [Google Scholar]
- 74.Yoshimura T, et al. Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature. 2003;426:178–181. doi: 10.1038/nature02117. [DOI] [PubMed] [Google Scholar]
- 75.Campos-Barros A, et al. Type 2 iodothyronine deiodinase expression in the cochlea before the onset of hearing. Proc. Natl Acad. Sci. USA. 2000;97:1287–1292. doi: 10.1073/pnas.97.3.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fekete C, Lechan RM. Central regulation of hypothalamic–pituitary–thyroid axis under physiological and pathophysiological conditions. Endocr. Rev. 2014;35:159–194. doi: 10.1210/er.2013-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Friesema EC, et al. Thyroid hormone transporters and deiodinases in the developing human hypothalamus. Eur. J. Endocrinol. 2012;167:379–386. doi: 10.1530/EJE-12-0177. [DOI] [PubMed] [Google Scholar]
- 78.Ng L, et al. Type 3 deiodinase, a thyroid-hormone-inactivating enzyme, controls survival and maturation of cone photoreceptors. J. Neurosci. 2010;30:3347–3357. doi: 10.1523/JNEUROSCI.5267-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kester MH, et al. Iodothyronine levels in the human developing brain: major regulatory roles of iodothyronine deiodinases in different areas. J. Clin. Endocrinol. Metab. 2004;89:3117–3128. doi: 10.1210/jc.2003-031832. [DOI] [PubMed] [Google Scholar]
- 80.Vose LR, et al. Treatment with thyroxine restores myelination and clinical recovery after intraventricular hemorrhage. J. Neurosci. 2013;33:17232–17246. doi: 10.1523/JNEUROSCI.2713-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zavacki AM, et al. Type 1 iodothyronine deiodinase is a sensitive marker of peripheral thyroid status in the mouse. Endocrinology. 2005;146:1568–1575. doi: 10.1210/en.2004-1392. [DOI] [PubMed] [Google Scholar]
- 82.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 83.Roti E, Fang SL, Green K, Emerson CH, Braverman LE. Human placenta is an active site of thyroxine and 3,3',5-triiodothyronine tyrosyl ring deiodination. J. Clin. Endocrinol. Metab. 1981;53:498–501. doi: 10.1210/jcem-53-3-498. [DOI] [PubMed] [Google Scholar]
- 84.Alexander EK, et al. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N. Engl. J. Med. 2004;351:241–249. doi: 10.1056/NEJMoa040079. [DOI] [PubMed] [Google Scholar]
- 85.Huang SA, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N. Engl. J. Med. 2000;343:185–189. doi: 10.1056/NEJM200007203430305. [DOI] [PubMed] [Google Scholar]
- 86.Abdalla SM, Bianco AC. Defending plasma T3 is a biological priority. Clin. Endocrinol. 2014;81:633–641. doi: 10.1111/cen.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J. Clin. Invest. 2006;116:2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steinsapir J, Harney J, Larsen PR. Type 2 iodothyronine deiodinase in rat pituitary tumor cells is inactivated in proteasomes. J. Clin. Invest. 1998;102:1895–1899. doi: 10.1172/JCI4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gereben B, Goncalves C, Harney JW, Larsen PR, Bianco AC. Selective proteolysis of human type 2 deiodinase: a novel ubiquitin-proteasomal mediated mechanism for regulation of hormone activation. Mol. Endocrinol. 2000;14:1697–1708. doi: 10.1210/mend.14.11.0558. [DOI] [PubMed] [Google Scholar]
- 90.Sagar GD, et al. Ubiquitination-induced conformational change within the deiodinase dimer is a switch regulating enzyme activity. Mol. Cell. Biol. 2007;27:4774–4783. doi: 10.1128/MCB.00283-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Egri P, Gereben B. Minimal requirements for ubiquitination-mediated regulation of thyroid hormone activation. J. Mol. Endocrinol. 2014;53:217–226. doi: 10.1530/JME-14-0156. [DOI] [PubMed] [Google Scholar]
- 92.Curcio-Morelli C, et al. In vivo dimerization of types 1, 2, and 3 iodothyronine selenodeiodinases. Endocrinology. 2003;144:937–946. doi: 10.1210/en.2002-220960. [DOI] [PubMed] [Google Scholar]
- 93.Arrojo EDR, Egri P, Jo S, Gereben B, Bianco AC. The type II deiodinase is retrotranslocated to the cytoplasm and proteasomes via p97/Atx3 complex. Mol. Endocrinol. 2013;27:2105–2115. doi: 10.1210/me.2013-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Curcio-Morelli C, et al. Deubiquitination of type 2 iodothyronine deiodinase by von Hippel-Lindau protein-interacting deubiquitinating enzymes regulates thyroid hormone activation. J. Clin. Invest. 2003;112:189–196. doi: 10.1172/JCI18348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Christoffolete MA, et al. Atypical expression of type 2 iodothyronine deiodinase in thyrotrophs explains the thyroxine-mediated pituitary thyrotropin feedback mechanism. Endocrinology. 2006;147:1735–1743. doi: 10.1210/en.2005-1300. [DOI] [PubMed] [Google Scholar]
- 96.Schneider MJ, et al. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol. Endocrinol. 2001;15:2137–2148. doi: 10.1210/mend.15.12.0740. [DOI] [PubMed] [Google Scholar]
- 97.Fekete C, Lechan RM. Negative feedback regulation of hypophysiotropic thyrotropin-releasing hormone (TRH) synthesizing neurons: role of neuronal afferents and type 2 deiodinase. Front. Neuroendocrinol. 2007;28:97–114. doi: 10.1016/j.yfrne.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boelen A, et al. Simultaneous changes in central and peripheral components of the hypothalamus–pituitary–thyroid axis in lipopolysaccharide-induced acute illness in mice. J. Endocrinol. 2004;182:315–323. doi: 10.1677/joe.0.1820315. [DOI] [PubMed] [Google Scholar]
- 99.Fekete C, et al. Lipopolysaccharide induces type 2 iodothyronine deiodinase in the mediobasal hypothalamus: implications for the nonthyroidal illness syndrome. Endocrinology. 2004;145:1649–1655. doi: 10.1210/en.2003-1439. [DOI] [PubMed] [Google Scholar]
- 100.Sawin CT, Surks MI, London M, Ranganathan C, Larsen PR. Oral thyroxine: variation in biologic action and tablet content. Ann. Intern. Med. 1984;100:641–645. doi: 10.7326/0003-4819-100-5-641. [DOI] [PubMed] [Google Scholar]
- 101.Ito M, et al. TSH-suppressive doses of levothyroxine are required to achieve preoperative native serum triiodothyronine levels in patients who have undergone total thyroidectomy. Eur. J. Endocrinol. 2012;167:373–378. doi: 10.1530/EJE-11-1029. [DOI] [PubMed] [Google Scholar]
- 102.Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012 ETA Guidelines: The use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur. Thyroid J. 2012;1:55–71. doi: 10.1159/000339444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone. J. Neuroendocrinol. 2008;20:784–794. doi: 10.1111/j.1365-2826.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- 104.Ritchie M, Yeap BB. Thyroid hormone: influences on mood and cognition in adults. Maturitas. 2015;81:266–275. doi: 10.1016/j.maturitas.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 105.Parsaik AK, et al. Hypothyroidism and risk of mild cognitive impairment in elderly persons: a population-based study. JAMA Neurol. 2014;71:201–207. doi: 10.1001/jamaneurol.2013.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wekking EM, et al. Cognitive functioning and well-being in euthyroid patients on thyroxine replacement therapy for primary hypothyroidism. Eur. J. Endocrinol. 2005;153:747–753. doi: 10.1530/eje.1.02025. [DOI] [PubMed] [Google Scholar]
- 107.Nasr A, Lauterio TJ, Davis MW. Unapproved drugs in the United States and the Food and Drug Administration. Adv. Ther. 2011;28:842–856. doi: 10.1007/s12325-011-0059-4. [DOI] [PubMed] [Google Scholar]
- 108.Markova N, et al. Hippocampal gene expression of deiodinases 2 and 3 and effects of 3,5-diiodo-L-thyronine T2 in mouse depression paradigms. Biomed. Res. Int. 2013;2013:565218. doi: 10.1155/2013/565218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Jong FJ, et al. Thyroid function, the risk of dementia and neuropathologic changes: the Honolulu-Asia aging study. Neurobiol. Aging. 2009;30:600–606. doi: 10.1016/j.neurobiolaging.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bauer M, Heinz A, Whybrow PC. Thyroid hormones, serotonin and mood: of synergy and significance in the adult brain. Mol. Psychiatry. 2002;7:140–156. doi: 10.1038/sj.mp.4000963. [DOI] [PubMed] [Google Scholar]
- 111.Morrissette DA, Stahl SM. Modulating the serotonin system in the treatment of major depressive disorder. CNS Spectr. 2014;19(Suppl. 1):57–67. doi: 10.1017/S1092852914000613. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Q, et al. Monitoring glutamate levels in the posterior cingulate cortex of thyroid dysfunction patients with TE-averaged PRESS at 3 T. Magn. Reson. Imaging. 2015;33:774–778. doi: 10.1016/j.mri.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 113.Schreckenberger MF, et al. Positron emission tomography reveals correlations between brain metabolism and mood changes in hyperthyroidism. J. Clin. Endocrinol. Metab. 2006;91:4786–4791. doi: 10.1210/jc.2006-0573. [DOI] [PubMed] [Google Scholar]
- 114.Hernandez A, Morte B, Belinchon MM, Ceballos A, Bernal J. Critical role of types 2 and 3 deiodinases in the negative regulation of gene expression by T3 in the mouse cerebral cortex. Endocrinology. 2012;153:2919–2928. doi: 10.1210/en.2011-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eravci M, Pinna G, Meinhold H, Baumgartner A. Effects of pharmacological and nonpharmacological treatments on thyroid hormone metabolism and concentrations in rat brain. Endocrinology. 2000;141:1027–1040. doi: 10.1210/endo.141.3.7358. [DOI] [PubMed] [Google Scholar]
- 116.Campos-Barros A, et al. The influence of desipramine on thyroid hormone metabolism in rat brain. J Pharmacol. Exp. Ther. 1994;268:1143–1152. [PubMed] [Google Scholar]
- 117.Baumgartner A, Dubeyko M, Campos-Barros A, Eravci M, Meinhold H. Subchronic administration of fluoxetine to rats affects triiodothyronine production and deiodination in regions of the cortex and in the limbic forebrain. Brain Res. 1994;635:68–74. doi: 10.1016/0006-8993(94)91424-9. [DOI] [PubMed] [Google Scholar]
- 118.Barez-Lopez S, et al. Abnormal motor phenotype at adult stages in mice lacking type 2 deiodinase. PLoS ONE. 2014;9:e103857. doi: 10.1371/journal.pone.0103857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J. Clin. Invest. 2006;116:476–484. doi: 10.1172/JCI26240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sittig LJ, Shukla PK, Herzing LB, Redei EE. Strain-specific vulnerability to alcohol exposure in utero via hippocampal parent-of-origin expression of deiodinase-III. FASEB J. 2011;25:2313–2324. doi: 10.1096/fj.10-179234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wilcoxon JS, Kuo AG, Disterhoft JF, Redei EE. Behavioral deficits associated with fetal alcohol exposure are reversed by prenatal thyroid hormone treatment: a role for maternal thyroid hormone deficiency in FAE. Mol. Psychiatry. 2005;10:961–971. doi: 10.1038/sj.mp.4001694. [DOI] [PubMed] [Google Scholar]
- 122.Wilcoxon JS, Redei EE. Prenatal programming of adult thyroid function by alcohol and thyroid hormones. Am. J. Physiol. Endocrinol. Metab. 2004;287:E318–E326. doi: 10.1152/ajpendo.00022.2004. [DOI] [PubMed] [Google Scholar]
- 123.Philibert RA, et al. The relationship of deiodinase 1 genotype and thyroid function to lifetime history of major depression in three independent populations. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156B:593–599. doi: 10.1002/ajmg.b.31200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cooper-Kazaz R, et al. Preliminary evidence that a functional polymorphism in type 1 deiodinase is associated with enhanced potentiation of the antidepressant effect of sertraline by triiodothyronine. J. Affect. Disord. 2009;116:113–116. doi: 10.1016/j.jad.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 125.Mentuccia D, et al. Association between a novel variant of the human type 2 deiodinase gene Thr92Ala and insulin resistance: evidence of interaction with the Trp64Arg variant of the β-3-adrenergic receptor. Diabetes. 2002;51:880–883. doi: 10.2337/diabetes.51.3.880. [DOI] [PubMed] [Google Scholar]
- 126.Estivalet AA, et al. D2 Thr92Ala and PPARγ2 Pro12Ala polymorphisms interact in the modulation of insulin resistance in type 2 diabetic patients. Obesity (Silver Spring) 2010;19:825–832. doi: 10.1038/oby.2010.231. [DOI] [PubMed] [Google Scholar]
- 127.Dora JM, Machado WE, Rheinheimer J, Crispim D, Maia AL. Association of the type 2 deiodinase Thr92Ala polymorphism with type 2 diabetes: case-control study and meta-analysis. Eur. J. Endocrinol. 2010;163:427–434. doi: 10.1530/EJE-10-0419. [DOI] [PubMed] [Google Scholar]
- 128.Guo TW, et al. Positive association of the DIO2 (deiodinase type 2) gene with mental retardation in the iodine-deficient areas of China. J. Med. Genet. 2004;41:585–590. doi: 10.1136/jmg.2004.019190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.He B, et al. Association of genetic polymorphisms in the type II deiodinase gene with bipolar disorder in a subset of Chinese population. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:986–990. doi: 10.1016/j.pnpbp.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 130.Taylor P, et al. Effect of low thyroid hormone bioavailability on childhood cognitive development: data from the Avon Longitudinal Study of Parents and Children birth cohort. Lancet. 2014;383(S100) [Google Scholar]
- 131.Galecka E, et al. Association of the DIO2 gene single nucleotide polymorphisms with recurrent depressive disorder. Acta Biochim. Pol. 2015;62:297–302. doi: 10.18388/abp.2015_1002. [DOI] [PubMed] [Google Scholar]
- 132.Canani LH, et al. The type 2 deiodinase A/G (Thr92Ala) polymorphism is associated with decreased enzyme velocity and increased insulin resistance in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2005;90:3472–3478. doi: 10.1210/jc.2004-1977. [DOI] [PubMed] [Google Scholar]
- 133.Peeters RP, et al. Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects. J. Clin. Endocrinol. Metab. 2003;88:2880–2888. doi: 10.1210/jc.2002-021592. [DOI] [PubMed] [Google Scholar]
- 134.Torlontano M, et al. Type 2 deiodinase polymorphism (threonine 92 alanine) predicts L-thyroxine dose to achieve target thyrotropin levels in thyroidectomized patients. J. Clin. Endocrinol. Metab. 2008;93:910–913. doi: 10.1210/jc.2007-1067. [DOI] [PubMed] [Google Scholar]
- 135.Butler PW, et al. The Thr92Ala 5' type 2 deiodinase gene polymorphism is associated with a delayed triiodothyronine secretion in response to the thyrotropin-releasing hormone-stimulation test: a pharmacogenomic study. Thyroid. 2011;20:1407–1412. doi: 10.1089/thy.2010.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang C, Kim S, Harney JW, Larsen PR. Further characterization of thyroid hormone response elements in the human type 1 iodothyronine deiodinase gene. Endocrinology. 1998;139:1156–1163. doi: 10.1210/endo.139.3.5849. [DOI] [PubMed] [Google Scholar]