Abstract
Although accumulating evidence indicates that heat shock protein 70 (HSP70) could be secreted into plasma and its levels have been found to have an ambiguous association with atherosclerosis, our knowledge for the exact role of circulating HSP70 in the development of atherosclerosis is still limited. In the present study, we report an adhesion-promoting effect of exogenous HSP70 and evaluate the potential involvement of elevated circulating HSP70 in the development of atherosclerosis. Time-dependent elevation of plasma HSP70 was found in diet-induced atherosclerotic rats, whose effect was investigated through further in vitro experiments. In rat aortic endothelial cell (RAEC) cultures, exogenous HSP70 incubation neither produced cell injuries by itself nor had protective effects on cell injuries caused by Ox-LDL or homocysteine. However, exogenous HSP70 administration could lead to a higher adhesion rate between rat peripheral blood monocytes (PBMCs) and RAECs. This adhesion-promoting effect appeared only when PBMCs, rather than RAECs, were pretreated with HSP70 incubation. PBMCs in an HSP70 environment released more IL-6 to supernatant, which subsequently up-regulated the expression of ICAM-1 in RAECs. These results indicate that the diet-induced elevation of circulating HSP70 could trigger cell adhesion with the help of IL-6 as a mediator, which provides a novel possible mechanism for understanding the role of circulating HSP70 in the pathogenesis of atherosclerosis.
Keywords: Circulating HSP70, Atherosclerosis, Exogenous HSP70, Cell adhesion, IL-6
Introduction
Atherosclerosis is a main cause of morbidity and mortality, the first stage of which was found to be an inflammatory response based on evidence from different laboratories (Ross 1999). Discoveries of adhesion molecule expression on endothelial cells and secretion of several cytokines have revealed an involvement of inflammatory and immune mechanisms in the pathogenesis of atherosclerosis (Libby et al. 2002; Wick and Xu 1999). In response to various stressors, vascular cells would produce a high level of heat shock proteins (HSPs) to block inflammations and maintain homeostasis during stress (House et al. 2001; Xu 2000, 2002). However, the immune reaction to HSPs was found to contribute to the development of atherosclerosis, which suggested that the induction of HSPs is beneficial in protecting the arterial wall against damage during stress but is harmful in certain other circumstances (Wick et al. 2014; Xu et al. 2012).
HSPs can be induced by a wide variety of stress stimuli and act as chaperones of proteins, protecting them from aggregation and improper folding (Hartl 1996). HSPs include several members belonging to six families, and the 70-kDa subgroup HSP70 is the most widely studied (Yoshida et al. 1990). HSP70 is normally located within cells to facilitate protein folding; however, it has also been found to be released in a soluble form from cells into the blood (Calderwood et al. 2007; Johnson et al. 1995). In contrast to the clearly cytoprotective action of its intracellular form, the role of circulating HSP70 is more complex and has been found to have an ambiguous association with atherosclerosis. Most investigators consider that the circulating HSP70 is the reflection of its intracellular expression and that it plays a similarly protective role against atherosclerosis development (Bielecka-Dabrowa et al. 2009). Epidemiological studies provide evidence of the inverse relationship between the level of plasma HSP70 and the progression of atherosclerosis (Martin-Ventura et al. 2007; Zhu et al. 2003). Functional studies have also reported that extracellular HSP70 could stimulate the production of anti-inflammatory cytokine IL-10 in responsive T cells (Wendling et al. 2000) and attenuate the intimal thickening in mice exposed to cigarette smoke (Matsumoto et al. 2008). In contrast, some conflicting reports showed the adverse effects of circulating HSP70 as a paracrine inducer of pro-inflammatory cytokines, especially IL-6, which exacerbate the inflammatory process in the development of atherosclerosis plaques (Asea et al. 2000; Gonzalez-Ramos et al. 2013; Svensson et al. 2006). HSP70 serum concentration was also shown to increase in patients with peripheral atherosclerosis according to some epidemiological investigations (Wright et al. 2000). Circulating HSP70 seems to be a pleiotropic molecule with complex biological actions in different microenvironments; however, its exact role in atherosclerosis is still unclear.
As a major cytokine in the pro-inflammatory scenario, IL-6 has been proved to be up-regulated by exogenous HSP70 (Asea et al. 2000) and plays a crucial role in the pathogenesis of atherosclerosis. Various studies have highlighted the central role of IL-6 as an upstream inflammatory cytokine in propagating the downstream inflammatory response responsible for atherosclerosis, such as endothelial cell activation and adhesion enhancement (Hartman and Frishman 2014; Watson et al. 1996). In this regard, IL-6 and its signaling events have been considered to contribute to both the atherosclerosis plaque development and destabilization via a variety of mechanisms (Schuett et al. 2009).
In the present study, we reported a novel adhesion-promoting effect of exogenous HSP70 and evaluated the potential involvement of circulating HSP70 in the development of atherosclerosis. The results indicated that a diet-induced elevation of plasma HSP70 would be likely to trigger cell adhesion between monocytes and endothelial cells with the help of IL-6 as a mediator, which provides a novel possible mechanism for understanding the role of circulating HSP70 in the pathogenesis of atherosclerosis.
Materials and methods
Animals
All animal experiments were performed on male SD rats weighing from 180 to 220 g, which were housed in plastic cages with access to food and water ad libitum and maintained on a 12-h light/dark cycle at 22–26 °C. The experimental protocols were approved by the Institutional Animal Care and Use Committee of IBMS (Permit Number: 2012-D-3098) and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023).
Induction of atherosclerosis and pathological study
Each experimental group consisted of 20 rats which were fed either a normal diet or a high-cholesterol diet for 12 weeks to induce atherosclerosis. The high-cholesterol diet contained 3 % cholesterol, 0.5 % cholic acid, 0.2 % 6-propyl 2-thiouracil, 5 % (w/w) lard, and 91.3 % regular rat chow, with the addition of a single dose of vitamin D3 (600,000 unit/kg, i.p.) (Sigma China, Beijing). Blood was collected every 2 weeks from the vena orbitalis posterior for plasma separation. Plasma total cholesterol (TC) and low-density lipoprotein (LDL) cholesterol were measured using corresponding kits (Sino-BioTech, China). At the end of the experimental 6, 8, 10, and 12 weeks, three rats were sacrificed for aorta collection under sodium pentobarbital (1 %, 50 mg/kg, i.p.) anesthesia. The separated aortas were maintained in −80 °C until western blot detection or were fixed in formalin and were prepared as paraffin sections for pathological analysis by hematoxylin and eosin staining.
Plasma HSP70 detection by western blot and Elisa assay
The alteration of HSP70 in plasma was assessed by western blot and Elisa assay. In western blot, the plasma samples were electrophoresed by SDS-PAGE gel and transferred onto a PVDF membrane with a Semidry Transfer System (Bio-Rad, USA). After blocking with 5 % milk in PBS, the membrane was incubated with anti-HSP70 antibody (H5147, Sigma-Aldrich, USA). The membranes were then incubated with horseradish peroxidase-conjugated secondary antibody (ZSGQ-Bio, China) for 2 h at room temperature. To normalize the loaded samples, mouse monoclonal anti-tubulin antibody (ZSGQ-Bio, China) was used, followed by incubation with horseradish peroxidase-conjugated anti-mouse IgG (ZSGQ-Bio, China). The membranes were incubated with Pierce ECL substrate kit (Thermo Scientific, USA), and images of the membranes were acquired with the ImageQuant LAS 4000 chemiluminescence imaging system (GE, USA) and were analyzed with Image Pro Plus software. The gray density of the band of HSP70 was measured and normalized to the gray density of the tubulin band. Plasma HSP70 release was also quantified using a Sandwich Elisa assay as previously described (Zhan et al. 2009). Briefly, microtiter plates with coated HSP70/HSP72 monoclonal antibody (ADI-SPA-810, Enzo Life Sciences, Switzerland) were incubated with recombinant rat HSP70/HSP72 (ADI-SPP-758, Enzo Life Sciences, Switzerland) or plasma samples. The plates were subsequently washed and incubated with rabbit anti-HSP70 antibody (sc-33575, Santa Cruz, USA). Peroxidase-conjugated anti-rabbit IgG and TMB substrate (ZSGQ-Bio, China) were used for chromogenic reaction. The reaction was terminated by H2SO4, and the absorbance was measured at 450 nm using a microplate reader (Thermo Fisher, USA). HSP70 concentrations in plasma were determined by comparing the absorbance to the standard curve which was constructed with recombinant rat HSP70/72.
Endothelial cell and monocytes culture
Rat aortic endothelial cells (RAECs) were prepared as described previously (Leng et al. 2013). RAECs were characterized by immunochemical staining using an antibody for endothelial cell-specific VIII factor relative antigen (sc-59957, Santa Cruz, USA). The endothelial cells were sub-cultured in medium-199 (Gibco, USA) supplemented with 10 % heat-inactivated fetal bovine serum (FBS, Invitrogen, USA), penicillin (100 U/ml), and streptomycin (100 mg/ml) in a humidified CO2 incubator. RAECs from the third and fifth passages were used for all the experiments. Sub-confluent cells were serum-starved for 24 h prior to the various treatments. Peripheral blood monocytes (PBMCs) were isolated from healthy adult SD rats using MACS cell separation kits (130-091-221, Miltenyi Biotec, Germany), and the purity was verified to be 95 % by morphological examination after cytostaining using an anti-CD14 antibody. The monocytes were maintained in RPMI-1640 (Gibco, USA) with 10 % FBS. All reagents were purchased from Sigma-Aldrich unless specified.
Lactate dehydrogenase assay
Cell injury was appraised quantitatively by measuring lactate dehydrogenase (LDH) release from damaged cells. The activity of LDH was determined spectrophotometrically at 340 nm using a commercial kit following the manufacturer’s instructions (Zhongsheng Co. China). Twenty-four-hour incubations of H2O2 (100 μM), Ox-LDL (50 μg/ml), or Hcy (5 mM) were used as positive controls to induce cell injury in different assays. Recombinant rat HSP70/HSP72 (ADI-SPP-758, Enzo Life Sciences, Switzerland) was administrated before or together with the Ox-LDL or Hcy incubation for the purpose of testing the potential protective role of exogenous HSP70.
MTT assay for cell survival rate evaluation
RAECs were seeded in a 96-well plate (5000 cell/well, 100 μl) for drug treatment; LPS (10 μg/ml) was set as a positive control. After 24 h of incubation, the medium was replaced as a 200-μl MTT medium (0.5 mg/ml) for another 4 h of incubation. Then, the MTT reagent was removed and 150 μl DMSO was added to each well with 15 min gentle shaking. The absorbance at 490 nm was read by a microplate reader. Cell survival rate was calculated as the ratio of absorbance between treatment and negative control. All experiments were performed in eight wells of each group and repeated three times.
Cell adhesion assay
RAECs at confluent status were treated with different doses of recombinant rat HSP70/HSP72 (ADI-SPP-758, Enzo Life Sciences, Switzerland) for 24 h and then co-cultured for another 30 min with freshly isolated PBMCs from healthy rats. Alternatively, normal PBMCs were treated with different doses of HSP70 for 24 h and then co-cultured with confluent RAECs for an additional 30 min. Control cells received only vehicle treatment. LPS (0.1 μg/ml)-treated cells were used as positive controls. Non-adherent cells were washed out using PBS with 1 % FBS. Attached PBMCs or RACEs were counted under an inverted microscope.
Detection for cell adhesion molecule and cytokine
Alteration of ICAM-1 expression in RAECs was quantified using a cell Elisa assay. In brief, RAECs were seeded in a 96-well plate (5000 cell/well, 100 μl) for HSP70 treatment; LPS (0.1 μg/ml) was set as a positive control. After 24 h of incubation, the medium containing HSP70 was replaced by 4 % paraformaldehyde for 15 min fixation. Three percent H2O2 and 10 % BSA in PBST were administrated by turns to block the endogenous peroxidase and nonspecific antibody-binding site, respectively. A 12-h incubation of goat anti-ICAM-1 antibody (sc-1511, Santa Cruz, USA) at 4 °C was used for ICAM-1 detection. Peroxidase-conjugated anti-goat IgG and TMB substrate (ZSGQ-Bio, China) were used for chromogenic at 405 nm. The levels of ICAM-1 were normalized by comparing optical densities to those in control wells. On the other hand, HSP70-induced IL-6 release from PBMCs was assessed by a Sandwich Elisa assay using a commercial kit (KRC0061, Thermo Fisher, USA) according to the manufacturer’s instructions.
Data analysis
Shapiro-Wilk normality test was first used to determine which data are normally or non-normally distributed. Normally distributed data were shown as mean ± SD, while non-normally distributed data were shown as median with interquartile range. Parametric one-way ANOVA followed by Fisher’s PLSD post hoc analysis was used for comparisons of the normally distributed data between groups. Non-parametric Kruskal-Wallis H test and Mann-Whitney U test were used for comparisons of non-normally distributed data (the level of plasma HSP70) between groups. All analysis was performed using the SPSS statistical package, and p < 0.05 was considered to be significantly different.
Results
Circulating HSP70 increased in diet-induced atherosclerotic rats
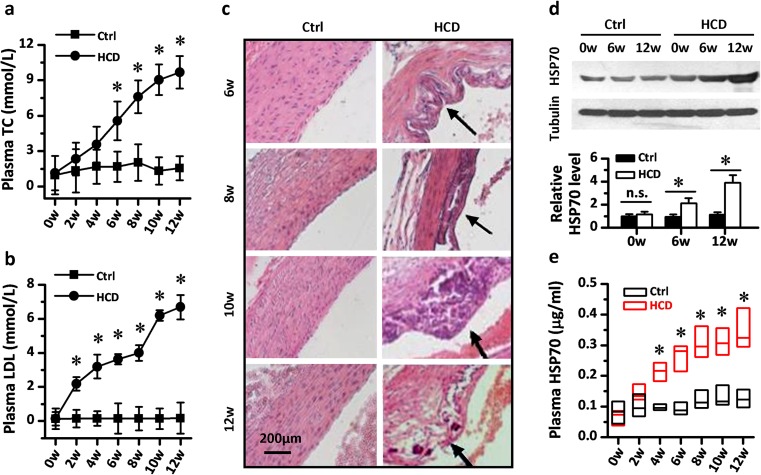
Numerous investigations have demonstrated that higher levels of plasma cholesterol could accelerate atherosclerosis and cause dysfunction in endothelial cells. In the present study, we fed SD rats with a high-cholesterol diet (HCD) for 12 weeks to induce atherosclerosis and monitored the alteration of circulating HSP70 levels in this pathogenesis process. As shown in Fig. 1a, b, rats that received HCD developed a time-dependent dysregulation in cholesterol metabolism. The level of plasma total cholesterol (TC) and low-density lipoprotein (LDL) cholesterol became significantly elevated from the sixth and the second week, respectively, in rats fed with HCD (p < 0.05 vs. control). And this higher level of TC and LDL increased linearly until the termination of the experiment. Pathological assessment was carried out from the sixth week, and the atherosclerotic changes and plaques similar to those in humans were observed in the aorta in the HCD group rats (Fig. 1c). To further characterize the possible role of plasma HSP70 in atherosclerosis, we measured the change of its level in HCD-induced atherosclerotic rats. Both western blot data and Elisa data indicated that a remarkable increase of plasma HSP70 concentration was induced by HCD in rats (Fig. 1d, e).
Fig. 1.
The level of circulating HSP70 went through a time-dependent elevation in diet-induced atherosclerotic rats. The rats that had received a high-cholesterol diet (HCD) developed a significant dysregulation in cholesterol metabolism. Higher levels of plasma total cholesterol (a) and plasma LDL (b) occurred from the sixth and the second week in the HCD group rats, n = 10. c Atherosclerotic pathological alterations appeared in the thoracic aorta of rats after 6-weeks of HCD feeding. The time-dependent elevation of circulating HSP70 was identified in the HCD group rats by western blot (d) and ELISA assay (e), respectively, n = 10. Western blot images were digitalized and analyzed by software. The gray density of the band of HSP70 was measured and normalized to the gray density of the tubulin band. ELISA results were shown as boxplots because of the non-normal distribution of HSP70 concentrations. Values in a–d represent mean ± SD. Horizontal lines across each box represent medians of plasma HSP70, while heights of each box represent interquartile ranges of HSP70 distribution in e. *p < 0.05 vs. Ctrl
Exogenous soluble HSP70 had no injurious or protective effects on cultured endothelial cells
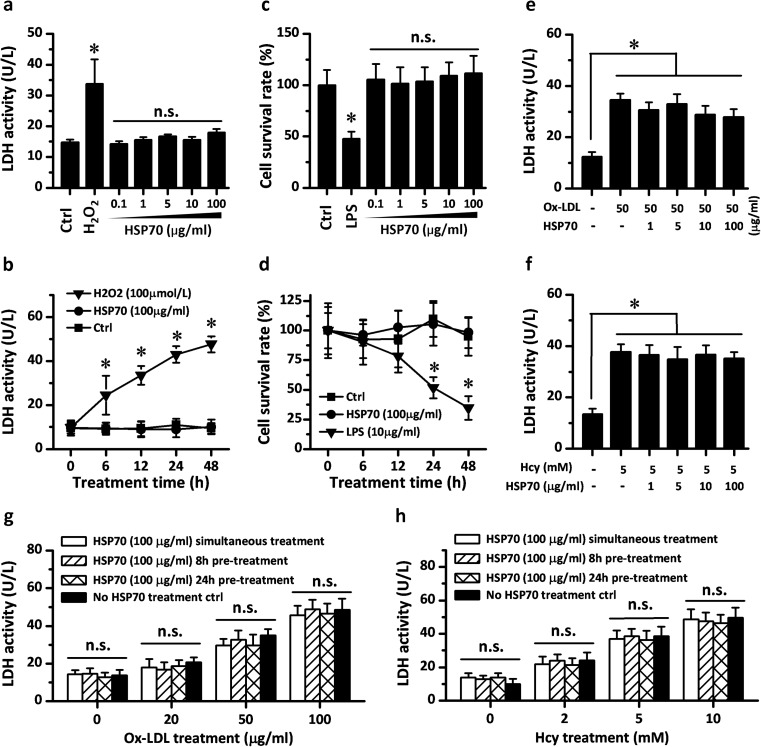
To study the relationship between the secreted circulating HSP70 and the development of atherosclerosis, the effects of exogenous HSP70 on cell activity were checked in primary endothelial cell cultures. In contrast to H2O2 treatment, HSP70 incubation with different concentrations or durations did not increase the activity of LDH in primary RAECs (Fig. 2a, b). Similarly, MTT assay showed that HSP70 incubation, throughout the doses or treatment times tested, did not result in alterations to cell survival rate compared with negative and positive controls (Fig. 2c, d), suggesting no direct injurious effects of HSP70 on RAECs culture. Subsequently, we examined whether exogenous HSP70 has protective effects on endothelial cells. Ox-LDL and Hcy, which could be detected in atherosclerotic plaque and cause endothelial dysfunction, were used as cell injury factors when incubated with HSP70. As shown in Fig. 2e–h, both Ox-LDL (50 μg/ml) and Hcy (5 mM) incubation for 24 h induced a significant increase in the LDH activity of cultured RAECs, while HSP70 administration with different concentrations or different modes (pretreatment or simultaneous treatment) failed to reverse the cell injury caused by Ox-LDL and Hcy.
Fig. 2.
Exogenous HSP70 neither induced injury or death in rat aortic endothelial cells (RAECs) nor had a cytoprotective role in cell injury in RAECs. Exogenous HSP70 was added with different doses and treatment times in the medium of primary cultured RAECs, while H2O2 and LPS were used as positive controls for cell injury and death, respectively. HSP70 did not induce any statistical change in cell LDH activity and survival rate in RAECs (a–d). The potential protective role of exogenous HSP70 was investigated in cell injury models. Different dose of recombinant HSP70 were added in the medium of RAECs simultaneously with the incubation of Ox-LDL or Hcy. The presence of HSP70 did not reverse the Ox-LDL or Hcy-induced increase in RAECs LDH activity (e–f). High dose of HSP70 was also administrated at times before the damaging compound. However, pretreatment with HSP70 for 8 or 24 h failed to decrease the level of LDH activity (g–h). Values represent mean ± SD, n = 3. *p < 0.05 vs. Ctrl
Exogenous HSP70 triggers cell adhesion by activating monocytes to release IL-6
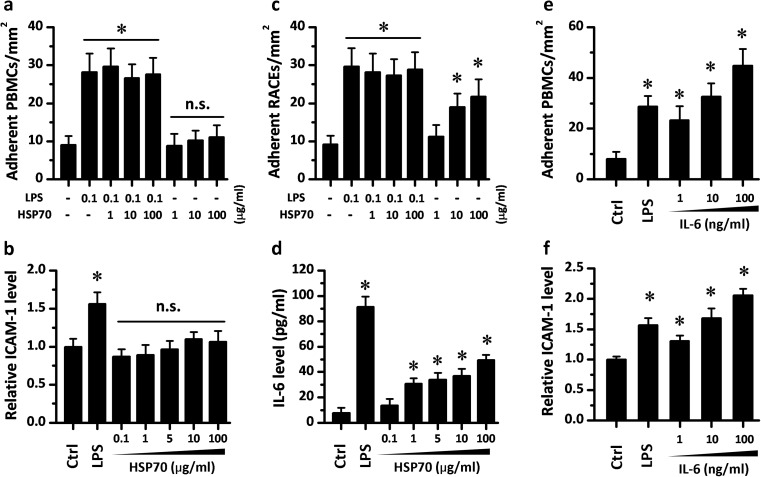
Then we examined whether exogenous HSP70 could regulate the interaction between monocytes and endothelial cells in vitro to reveal the potential physiological significance of circulating HSP70. RAECs with or without pretreatment of HSP70 were co-cultured with PBMCs. No significant change was observed in the density of adherent PBMCs among groups, which suggested that HSP70 treatment alone could not activate endothelial cells (Fig. 3a). In addition, HSP70 had no impact on the adhesion of PBMCs to RAECs which were activated by LPS (Fig. 3a), Ox-LDL, and Hcy (data not shown). Consistent with these results, 24-h HSP70 incubation also failed to induce higher expression of ICAM-1 in RAECs (Fig. 3b). However, when PBMCs were pretreated with HSP70 at doses higher than 10 μg/ml, they attracted more normal RAECs that adhered to them (note the higher density of adherent RAECs in Fig. 3c). To further explore the mechanisms underlying the adhesion-promoting role of HSP70, we also measured the cytokine release in PBMCs. An Elisa assay for IL-6 indicated that HSP70 treatment could induce a dose-dependent secretion of IL-6 in PBMCs (Fig. 3d). IL-6 treatment could also activate RAECs and result in higher expression of ICAM-1 in RAECs (Fig. 3f) and a higher adhesion rate of PBMCs to RAECs, which was similar to the situation of HSP70 pretreatment in PBMCs (Fig. 3e). These results suggest that exogenous HSP70 might provide a paracrine regulation of the interaction between monocytes and endothelial cells in the vasculature.
Fig. 3.
Exogenous HSP70 promotes the adhesion between rat peripheral blood monocytes (PBMCs) and rat aortic endothelial cells (RAECs) by cytokine release. a RAECs were treated with LPS and HSP70 for 24 h and then incubated with freshly isolated PBMC for an additional 30 min. Adherent PBMCs density indicated that HSP70 has no effect on the adhesion of RAECs. Consistently, 24 h HSP70 treatment failed to induce a higher expression of ICAM-1 in RAECs (b). However, when the experimental procedure was reversed, HSP70 treatment on PBMCs resulted in a higher adhesion rate between PBMCs and RAECs (c). d 24-h HSP70 incubation also induced higher IL-6 release in PBMCs. e RAECs were treated with LPS and IL-6 for 24 h and then incubated with PBMCs for 30 min. Similar adhesion-promoting effects of IL-6 were indicated by higher adherent PBMCs density. f 24-h IL-6 treatment on RAECs led to a dramatic up-regulation of ICAM-1. Values represent mean ± SD, n = 3. *p < 0.05 vs. Ctrl
Discussion
HSP70, also known as HSPA1A in humans, serving as both molecular chaperones and cytokines, not only play a role in cell protection from damage in response to stress stimuli but are also involved in the pathogenesis of some diseases (Kaiser et al. 2014; Xu et al. 2012). In the present study, we identified a time-dependent elevation of plasma HSP70 in HCD-induced atherosclerotic rats and demonstrated that exogenous HSP70 could activate monocytes to promote the adhesion between endothelial cells and monocytes. These results indicated that a diet-induced release of HSP70 (HSPA1A) might act as a paracrine factor to regulate vascular homeostasis.
A HCD is a commonly used model to evoke atherosclerosis in animals (Cai et al. 2005). As in our previous studies (Leng et al. 2010, 2013), we successfully induced atherosclerotic pathological changes in rats’ thoracic aorta accompanied by a significant elevation of plasma TC, LDL, Hcy, and most importantly by circulating HSP70. The increased plasma HSP70 probably derived from multiple cells. Our previous experiments had proved that the endothelial cell could release HSP70 to plasma by an exosome-dependent pathway in the presence of Ox-LDL or Hcy (Zhan et al. 2009). Ox-LDL could also induce the secretion of HSP70 from intact macrophages into the blood (Hunter-Lavin et al. 2004; Svensson et al. 2006). Mast cells, dendrite cells, and even tumor cells had also been observed to be a source of circulating HSP70s (Laulagnier et al. 2004; Mambula and Calderwood 2006). Our result of elevated plasma HSP70 is consistent with the data from Krepuska (Krepuska et al. 2011) and Wright (Wright et al. 2000), which showed an increased level of serum HSP70 (HSPA1A) in atherosclerosis patients, but is in contrast to other reports showing a lower concentration of circulating HSP70 (HSPA1A) in atherosclerotic subjects (Dulin et al. 2010; Galovic et al. 2016). The reasons for this conflict have not been fully understood, but perhaps the differences in the models and species may contribute to explain the contrasting findings. The conflict may also reflect the complex biological actions of soluble HSP70 in different microenvironments.
The endothelium is the major regulator of vascular homeostasis, whose dysfunction is an early marker for atherosclerosis (Deanfield et al. 2007). Soluble HSP70 had been found to act as a pro-inflammatory cytokine binding to Toll-like receptors (TLRs) of monocytes to exaggerate the pathogenesis of vascular diseases (Asea et al. 2002). Vascular endothelial cells express TLRs under baseline conditions, and the expression of TLR2 and TLR4 would increase substantially in the endothelium covering atherosclerotic lesions (Edfeldt et al. 2002; Faure et al. 2001). Thus, the endothelial cells might be a potential target of circulating HSP70. We evaluated the effects of exogenous recombinant rat HSP70 on RAECs by in vitro experiment. Surprisingly, we failed to detect either the direct injurious effects or the protective effects of HSP70 on cultured RAECs. Moreover, the lower adhesion rate of PBMCs to RAECs and the unchanged expression of ICAM-1 and IL-6 suggested that although endothelial cells expressed TLRs, they cannot be activated by HSP70 alone. These results were likely attributed to the different specific characters of different cells. In addition, it was reported that CD14 was necessary for the effects of soluble HSP70 binding to TLR4 on NF-kB activation (Asea et al. 2000; Dybdahl et al. 2002). The lack of sufficient CD14 in the culture environment would be another possible reason for the insensitivity of endothelial cells to HSP70.
Endothelial cells are not the only potential target of circulating HSP70. Monocytes, natural killer cells, and dendrite cells were also found to be activated by soluble HSP70 or HSP60 and to produce more pro-inflammatory factors such as TNF-α and IL-8 (Gross et al. 2003; Lipsker et al. 2002; Yokota et al. 2006). Thus, we also evaluated the role of HSP70 in monocytes and found that exogenous HSP70 administration led to a higher adhesion rate between PBMCs and RAECs. It is known that elevations of intracellular HSP70 in cells of the blood vessel walls decrease the expression of inflammatory factors and inhibit the cell adhesion between endothelium and leukocyte (House et al. 2001; Kohn et al. 2002). However, our data implied that HSP70 would have a dramatic different effect on PBMCs to promote cell adhesion when it released outside of endothelial cells and circulated in the blood. On the other hand, the present study also implied that the HSP70-evoked selective adhesion-promoting effect on monocytes would be probably mediated by IL-6 release. Pretreatment with exogenous HSP70 incubation caused only PBMCs, rather than RAECs, to produce more IL-6, which could subsequently up-regulate the expression of ICAM-1 in RAECs and mimic the adhesion-promoting effects of exogenous HSP70 on monocytes. Various studies have highlighted the central role of IL-6 as an upstream inflammatory cytokine in propagating the downstream inflammatory response responsible for atherosclerosis, such as endothelial cell activation and adhesion enhancement (Hartman and Frishman 2014; Watson et al. 1996). Considering the initial role of cell adhesion in the development of atherosclerosis, we speculated that the secreted circulating HSP70 (HSPA1A) might act as a pro-atherosclerosis factor to trigger cell adhesion between monocytes and endothelial cells with the help of IL-6, which provides a novel possible mechanism for understanding the pathogenesis of atherosclerosis. The exact physiological and pathological significance of circulating HSP70 (HSPA1A) in vascular lesion formation and atherosclerosis development will require further investigation.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81401041, No. 81302602, No. 31571173, No. 31400948, and No. 81500454).
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no competing interests.
Footnotes
Fang Xie and Rui Zhan contributed equally to this work.
References
- Asea A, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Asea A, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Bielecka-Dabrowa A, Barylski M, Mikhailidis DP, Rysz J, Banach M. HSP 70 and atherosclerosis—protector or activator? Expert Opin Ther Targets. 2009;13:307–317. doi: 10.1517/14728220902725149. [DOI] [PubMed] [Google Scholar]
- Cai GJ, Miao CY, Xie HH, Lu LH, Su DF. Arterial baroreflex dysfunction promotes atherosclerosis in rats. Atherosclerosis. 2005;183:41–47. doi: 10.1016/j.atherosclerosis.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Mambula SS, Gray PJ, Jr, Theriault JR. Extracellular heat shock proteins in cell signaling. FEBS Lett. 2007;581:3689–3694. doi: 10.1016/j.febslet.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- Dulin E, Garcia-Barreno P, Guisasola MC. Extracellular heat shock protein 70 (HSPA1A) and classical vascular risk factors in a general population. Cell Stress Chaperones. 2010;15:929–937. doi: 10.1007/s12192-010-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybdahl B, et al. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. 2002;105:685–690. doi: 10.1161/hc0602.103617. [DOI] [PubMed] [Google Scholar]
- Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- Faure E, Thomas L, Xu H, Medvedev A, Equils O, Arditi M. Bacterial lipopolysaccharide and IFN-gamma induce Toll-like receptor 2 and Toll-like receptor 4 expression in human endothelial cells: role of NF-kappa B activation. J Immunol. 2001;166:2018–2024. doi: 10.4049/jimmunol.166.3.2018. [DOI] [PubMed] [Google Scholar]
- Galovic R, Flegar-Mestric Z, Vidjak V, Matokanovic M, Barisic K. Heat shock protein 70 and antibodies to heat shock protein 60 are associated with cerebrovascular atherosclerosis. Clin Biochem. 2016;49:66–69. doi: 10.1016/j.clinbiochem.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Ramos M, et al. HSP70 increases extracellular matrix production by human vascular smooth muscle through TGF-beta1 up-regulation. Int J Biochem Cell Biol. 2013;45:232–242. doi: 10.1016/j.biocel.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Gross C, Hansch D, Gastpar R, Multhoff G. Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biol Chem. 2003;384:267–279. doi: 10.1515/BC.2003.030. [DOI] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Hartman J, Frishman WH. Inflammation and atherosclerosis: a review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev. 2014;22:147–151. doi: 10.1097/CRD.0000000000000021. [DOI] [PubMed] [Google Scholar]
- House SD, et al. Effects of heat shock, stannous chloride, and gallium nitrate on the rat inflammatory response. Cell Stress Chaperones. 2001;6:164–171. doi: 10.1379/1466-1268(2001)006<0164:EOHSSC>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter-Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM, Williams JH. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–517. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- Johnson AD, Berberian PA, Tytell M, Bond MG. Differential distribution of 70-kD heat shock protein in atherosclerosis. Its potential role in arterial SMC survival. Arterioscler Thromb Vasc Biol. 1995;15:27–36. doi: 10.1161/01.ATV.15.1.27. [DOI] [PubMed] [Google Scholar]
- Kaiser F, Steptoe A, Thompson S, Henderson B. Monocyte cytokine synthesis in response to extracellular cell stress proteins suggests these proteins exhibit network behaviour. Cell Stress Chaperones. 2014;19:135–144. doi: 10.1007/s12192-013-0440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn G, et al. Heat shock inhibits tnf-induced ICAM-1 expression in human endothelial cells via I kappa kinase inhibition. Shock. 2002;17:91–97. doi: 10.1097/00024382-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Krepuska M, et al. Serum level of soluble Hsp70 is associated with vascular calcification. Cell Stress Chaperones. 2011;16:257–265. doi: 10.1007/s12192-010-0237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laulagnier K, et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J. 2004;380:161–171. doi: 10.1042/bj20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng X, et al. Anti-heat shock protein 70 autoantibody epitope changes and BD091 promotes atherosclerosis in rats. Cell Stress Chaperones. 2010;15:947–958. doi: 10.1007/s12192-010-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng X, et al. Evidence of a role for both anti-Hsp70 antibody and endothelial surface membrane Hsp70 in atherosclerosis. Cell Stress Chaperones. 2013;18:483–493. doi: 10.1007/s12192-013-0404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Lipsker D, et al. Heat shock proteins 70 and 60 share common receptors which are expressed on human monocyte-derived but not epidermal dendritic cells. Eur J Immunol. 2002;32:322–332. doi: 10.1002/1521-4141(200202)32:2<322::AID-IMMU322>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849–7857. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- Martin-Ventura JL, Leclercq A, Blanco-Colio LM, Egido J, Rossignol P, Meilhac O, Michel JB. Low plasma levels of HSP70 in patients with carotid atherosclerosis are associated with increased levels of proteolytic markers of neutrophil activation. Atherosclerosis. 2007;194:334–341. doi: 10.1016/j.atherosclerosis.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, et al. Exogenous heat shock protein-70 inhibits cigarette smoke-induced intimal thickening. Am J Physiol Regul, Integr Comp Physiol. 2008;295:R1320–1327. doi: 10.1152/ajpregu.00624.2007. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Schuett H, Luchtefeld M, Grothusen C, Grote K, Schieffer B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb Haemost. 2009;102:215–222. doi: 10.1160/TH09-05-0297. [DOI] [PubMed] [Google Scholar]
- Svensson PA, et al. Major role of HSP70 as a paracrine inducer of cytokine production in human oxidized LDL treated macrophages. Atherosclerosis. 2006;185:32–38. doi: 10.1016/j.atherosclerosis.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C, Whittaker S, Smith N, Vora AJ, Dumonde DC, Brown KA. IL-6 acts on endothelial cells to preferentially increase their adherence for lymphocytes. Clin Exp Immunol. 1996;105:112–119. doi: 10.1046/j.1365-2249.1996.d01-717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling U, Paul L, van der Zee R, Prakken B, Singh M, van Eden W. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-hsp70 homologue. J Immunol. 2000;164:2711–2717. doi: 10.4049/jimmunol.164.5.2711. [DOI] [PubMed] [Google Scholar]
- Wick G, Xu Q. Atherosclerosis—an autoimmune disease. Exp Gerontol. 1999;34:559–566. doi: 10.1016/S0531-5565(99)00035-2. [DOI] [PubMed] [Google Scholar]
- Wick G, Jakic B, Buszko M, Wick MC, Grundtman C. The role of heat shock proteins in atherosclerosis. Nat Rev Cardiol. 2014;11:516–529. doi: 10.1038/nrcardio.2014.91. [DOI] [PubMed] [Google Scholar]
- Wright BH, Corton JM, El-Nahas AM, Wood RF, Pockley AG. Elevated levels of circulating heat shock protein 70 (Hsp70) in peripheral and renal vascular disease. Heart Vessel. 2000;15:18–22. doi: 10.1007/s003800070043. [DOI] [PubMed] [Google Scholar]
- Xu Q. Biomechanical-stress-induced signaling and gene expression in the development of arteriosclerosis. Trends Cardiovasc Med. 2000;10:35–41. doi: 10.1016/S1050-1738(00)00042-6. [DOI] [PubMed] [Google Scholar]
- Xu Q. Role of heat shock proteins in atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:1547–1559. doi: 10.1161/01.ATV.0000029720.59649.50. [DOI] [PubMed] [Google Scholar]
- Xu Q, Metzler B, Jahangiri M, Mandal K. Molecular chaperones and heat shock proteins in atherosclerosis. Am J Physiol Heart Circ Physiol. 2012;302:H506–514. doi: 10.1152/ajpheart.00646.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Minota S, Fujii N. Anti-HSP auto-antibodies enhance HSP-induced pro-inflammatory cytokine production in human monocytic cells via Toll-like receptors. Int Immunol. 2006;18:573–580. doi: 10.1093/intimm/dxh399. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Shiga T, Imai S. Degradation of sarcoplasmic reticulum calcium-pumping ATPase in ischemic-reperfused myocardium: role of calcium-activated neutral protease. Basic Res Cardiol. 1990;85:495–507. doi: 10.1007/BF01931495. [DOI] [PubMed] [Google Scholar]
- Zhan R, et al. Heat shock protein 70 is secreted from endothelial cells by a non-classical pathway involving exosomes. Biochem Biophys Res Commun. 2009;387:229–233. doi: 10.1016/j.bbrc.2009.06.095. [DOI] [PubMed] [Google Scholar]
- Zhu J, et al. Increased serum levels of heat shock protein 70 are associated with low risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2003;23:1055–1059. doi: 10.1161/01.ATV.0000074899.60898.FD. [DOI] [PubMed] [Google Scholar]