Abstract
Large bowel carcinogenesis involves accumulation of genetic alterations leading to transformation of normal mucosa into dysplasia and, lastly, adenocarcinoma. It is pertinent to elucidate the molecular changes occurring in the pre-neoplastic lesions to facilitate early diagnosis and treatment. Heat shock proteins (Hsps), many of which are molecular chaperones, are implicated in carcinogenesis, and their variations with tumor progression encourage their study as biomarkers. There are many reports on Hsps and cancer but none to our knowledge on their systematic quantification in pre-neoplastic lesions of the large bowel. We performed immunohistochemical determinations of Hsp10, Hsp60, Hsp70, and Hsp90 in biopsies of large bowel tubular adenomas with moderate grade of dysplasia and compared to normal mucosa and adenocarcinoma with a moderate grade of differentiation (G2). A significant elevation of Hsp10 and Hsp60 only, i.e., in the absence of elevation of Hsp70 or Hsp90, in both epithelium and lamina propria was found in tubular adenoma by comparison with normal mucosa. In contrast, adenocarcinoma was characterized by the highest levels of Hsp10 and Hsp60 in epithelium and lamina propria, accompanied by the highest levels of Hsp70 only in epithelium and of Hsp90 only in lamina propria, by comparison with normal and tubular adenoma counterparts. Hsp10 and Hsp60 are promising biomarkers for early diagnosis of tubular adenoma and for its differentiation from more advanced malignant lesions. Hsp10 and Hsp60 may be implicated in carcinogenesis from its very early steps and, thus, are potentially convenient targets for therapy.
Keywords: Hsps, Chaperone, Large bowel, Dysplasia, Tubular adenoma, Biomarker
Introduction
The carcinogenic process of large bowel consists of a multistep sequence in which the transformation of normal large bowel mucosa into an invasive tumor involves the accumulation of various genetic alterations (Vogelstein et al. 1988), which are accompanied by typical histological patterns visible under the microscope. However, it is frequently challenging for the pathologist to determine at which step in the carcinogenic sequence is a tissue sample from a patient. Therefore, the search for reliable, ideally step-specific, biomarkers is worthwhile. Some heat shock proteins (Hsps) are biomarker candidates since they have been found to vary in quantity and distribution during carcinogenesis (Cappello et al. 2005a; Rappa et al. 2012; Campanella et al. 2015). In this work, we have focused on Hsp10, Hsp60, Hsp70, and Hsp90 to examine their diagnostic value in tubular adenoma and, thereby, to determine their utility in assessing the prognosis of an intestinal lesion not yet clearly malignant.
Many Hsps are part of the chaperoning system, a physiological system that is essential for protein homeostasis (Macario and Conway de Macario 2005). Hsps perform also other important functions such as participation in immune system regulation (Pockley et al. 2008), cell differentiation (Walsh et al. 1999), gene expression (Voellmy 1994), programmed cell death (Kirchhoff et al. 2002), cellular senescence (Di Felice et al. 2005), and carcinogenesis (Cappello and Zummo 2005b; Czarnecka et al. 2006a, b). Some Hsps, including Hsp10, Hsp60, Hsp70, and Hsp90 are constitutively expressed in cells (Macario and Conway de Macario 2005).
The implication of Hsps in the carcinogenic process includes their participation in cell proliferation (Czarnecka et al. 2006a), angiogenesis (Sanderson et al. 2006), invasiveness (Zhao et al. 2007), and induction of immune tolerance (Calderwood et al. 2005). Several studies have shown that elevated levels of Hsps can protect malignant cells against apoptosis induced by therapy (Joly et al. 2010).
In the past, we studied the levels of Hsp10, Hsp60, and Hsp90 in adenocarcinoma of human large bowel (Cappello et al. 2005c; Rappa et al. 2014; Campanella et al. 2015). In the present work, we focused on the immunohistochemical levels of Hsp10, Hsp60, Hsp70, and Hsp90 in large bowel tubular adenomas with moderate grade of dysplasia and compared to normal mucosa and to adenocarcinoma with a moderate grade of differentiation (G2). Our aim was to determine if these molecules are useful as biomarkers for early diagnosis of pre-tumoral lesions in the large bowel.
Materials and methods
Formalin-fixed paraffin-embedded tissue biopsies of human colorectal normal mucosa, tubular adenomas with moderate grade of dysplasia, and adenocarcinoma with a moderate grade of differentiation (G2) (n = 60 cases for each group) were retrieved from our histological specimens collection for immunohistochemical analyses. The normal mucosa group consisted of 60 subjects (40 male and 20 female; average age 65 ± 4 years) who underwent colorectal endoscopy for screening. These subjects did not show any pathological condition (cancer, polyps, or inflammatory signs). The tubular adenomas group consisted of 60 patients. Thirty-six (60 %) were men and twenty-four (40 %) were women. The average age of patients was 64.9 ± 9.4 (50–87 years). The average size of the polyps was 0.9 ± 0.2 cm (0.5–1.4 cm); in particular, the polyps were <1 cm in size in 31 patients and ≥1 cm in 29 patients. The polyps were located in the colon in 46 cases (18 in the right colon and 28 in the left colon) and in the rectum in 14 cases. All polyps selected were histologically tubular adenomas and single for each patient. The adenocarcinoma group consisted of 60 patients affected by colorectal adenocarcinoma, 33 (55 %) were men and 27 (45 %) were women. The average age of patients was 65.8 ± 8.6 (49–89 years). All tumor samples selected were histologically adenocarcinoma with moderate grade of differentiation (G2). The tumor localization was right colon in 20 cases, left colon in 28 cases, and rectum in 12 cases. The tumoral staging was stage I in 18 cases, stage II in 32 cases, and stage III in 10 cases. Studies of genetic mutation were not performed. Sections with a thickness of 4–5 μm were obtained from paraffin blocks of biopsies with a cutting microtome. These sections were dewaxed in xylene for 30 min at 60 °C and after immersion in a descending scale of alcohols, rehydrated in distiller water at 23 °C. After deparaffination, antigen unmasking was performed with immersion of sections in sodium citrate buffer (pH 6) at 95 °C for 8 min and, later, with immersion in acetone at −20 °C for 8 min. All subsequent reactions were conducted at 23 °C. After a wash with PBS (phosphate buffered saline pH 7.4) for 5 min, sections were immunostained, using Histostain®-Plus 3rd Gen IHC Detection Kit (Life Techologies, Frederik, MD, USA; Cat. No. 85–9073), which utilizes the labeled biotin-streptavidin methodology. The primary antibodies used were anti-human Hsp10 (rabbit polyclonal antibody, clone FL-102, Santa Cruz Biotechnology, Inc., Heidelberg, Germany, Europe, cat. no. Sc-28,887, dilution 1:200), anti-human Hsp60 (mouse monoclonal antibody, clone LK1, Sigma, St. Louis, MO, USA, Cat. No. H4149, dilution 1:400), anti-human Hsp70/HSC70 (mouse monoclonal antibody, clone W27, Santa Cruz Biotechnology, Inc., Europe, Cat. No. sc-24, dilution 1:200), and anti-human Hsp90 (mouse monoclonal antibody, clone F-8, Santa Cruz Biotechnology, Inc., Europe, Cat. No. sc-13,119, dilution 1:200). Appropriate negative (isotype) controls were run concurrently. Nuclear counterstaining was done using hematoxylin (Hematoxylin aqueous formula, DAKO, Denmark, N. Cat. CS 700). Finally, the sections were observed with an optical microscope (Nikon ECLIPSE Ni, Nikon Instrument Europe B.V.) connected to a digital camera (DS-Fi2, Nikon Instrument Europe B.V.) for the immunostaining evaluation. Two independent observers (F. C and F. R) examined the specimens on two separate occasions and performed a quantitative analysis to determine the percentage of cells positive for Hsp10, Hsp60, Hsp70, Hsp90 in epithelium and lamina propria of colon mucosa and to find possible correlations between immunopositivity of Hsps and size and anatomical location in the tubular adenoma group and location and staging in the adenocarcinoma group. All the observations were made at a magnification of 400×, and the percentage of positive cells was calculated in a high-power field (HPF) and repeated for 10 HFP. The arithmetic means of counts were used for statistical analyses. Statistical analyses were carried out using the GraphPad Prism 4.0 package (GraphPad Inc., San Diego, CA, USA). One-way ANOVA analysis of variance with Bonferroni post-hoc multiple comparisons was used to find significant statistical differences. All data are presented as the means ± SD, and the threshold level of statistical significance was set at p ≤ 0.05, as indicated in the text and in the figures.
Results
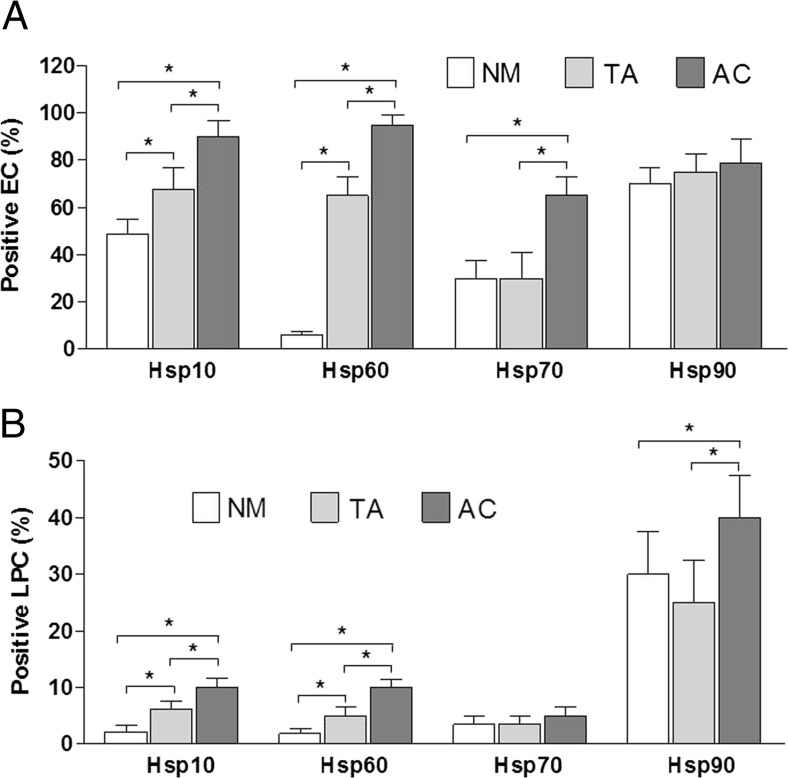
Hsp10, Hsp60, Hsp70, and Hsp90 were detected and quantified immunohistochemically in epithelial and lamina propria in biopsies of colon normal mucosa, and tubular adenoma and adenocarcinoma counterparts. The immunopositivity evaluations are expressed as average percentage in epithelial cells (EC) and lamina propria cells (LPC) and are reported in Table 1 and Fig. 1; representative images are displayed in Figs. 2 and 3. In summary, in the normal mucosa group, Hsp10 immunopositivity was cytoplasmic in 48.5 % EC and 2.1 % LPC; Hsp60 immunopositivity was cytoplasmic and granular in 6 % EP and 1.9 % LPC; Hsp70 immunopositivity was cytoplasmic in 30 % EC and 3.5 % LPC; Hsp90 immunopositivity was cytoplasmic in 70 % EC and 30 % LPC. By contrast, in the tubular adenoma group, Hsp10 immunopositivity was cytoplasmic in 67.5 % EC and 6.2 % of LPC; Hsp60 immunopositivity was cytoplasmic and granular in 65 % EP and 5 % LPC; Hsp70 immunopositivity was cytoplasmic in 30 % EC and 3.5 % LPC; Hsp90 immunopositivity was cytoplasmic in 75 % EC and 25 % LPC. In the adenocarcinoma group, Hsp10 immunopositivity was cytoplasmic in 90 % EC and 10 % LPC; Hsp60 immunopositivity was visible at cytoplasmic and membrane levels in 95 % EP and 10 % LPC; Hsp70 immunopositivity was cytoplasmic and sometimes also nuclear in 65 % EC and 5 % LPC; Hsp90 immunopositivity was cytoplasmic in 78 % EC and 40 % LPC.
Table 1.
Percentages of cells immunopositive for Hsps in large bowel mucosa
| Immunostaining for: | NM | TA | AC | |||
|---|---|---|---|---|---|---|
| EC (%) | LPC (%) | EC (%) | LPC (%) | EC (%) | LPC (%) | |
| Hsp10 | 48.5 | 2.1 | 67.5 | 6.2 | 90 | 10 |
| Hsp60 | 6 | 1.9 | 65 | 5 | 95 | 10 |
| Hsp70 | 30 | 3.5 | 30 | 3.5 | 65 | 5 |
| Hsp90 | 70 | 30 | 75 | 25 | 78 | 40 |
The immunohistochemical evaluations are expressed as average percentage in epithelial cells (EC) and lamina propria cells (LPC) in normal mucosa (NM), tubular adenoma (TA), and adenocarcinoma (AC) of human large bowel (see Materials and Methods for technical details)
Fig. 1.
Histograms show percentage of cells immunopositive for Hsp10, Hsp60, Hsp70, and Hsp90 in epithelial cells (EC) (a) and in cells of lamina propria (LPC) (b) in human large bowel normal mucosa (NM), tubular adenoma (TA), and adenocarcinoma (AC). Data are presented as the means ± SD. *p < 0.05
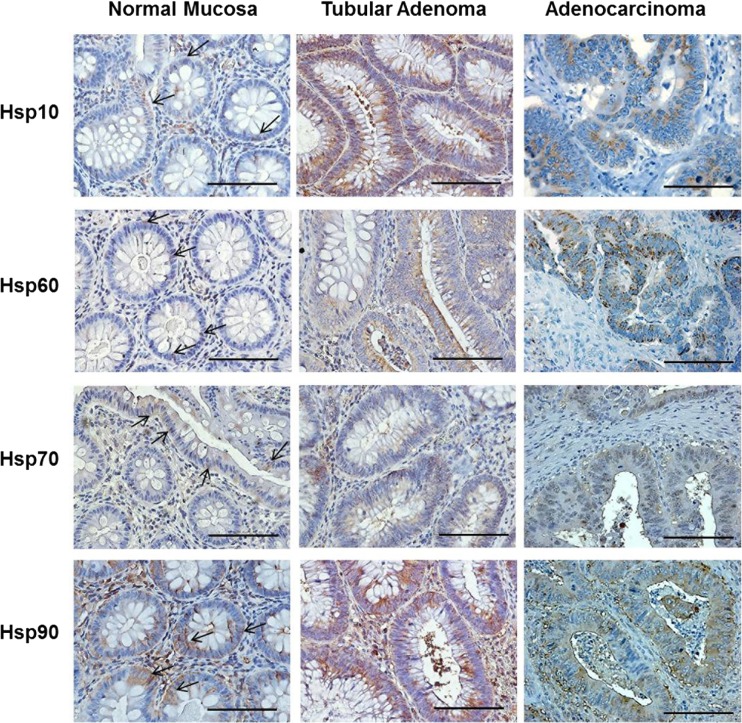
Fig. 2.
Representative images of immunohistochemical results for Hsp10, Hsp60, Hsp70, and Hsp90 in human large bowel biopsies of normal mucosa, tubular adenoma with moderate grade of dysplasia, and adenocarcinoma with moderate grade of differentiation. Magnification 400×. Scale bar 100 μm. Arrows show the positivity for the Hsps in the epithelial cells of normal mucosa. Note the slight positivity for Hsp60 in normal epithelial cells
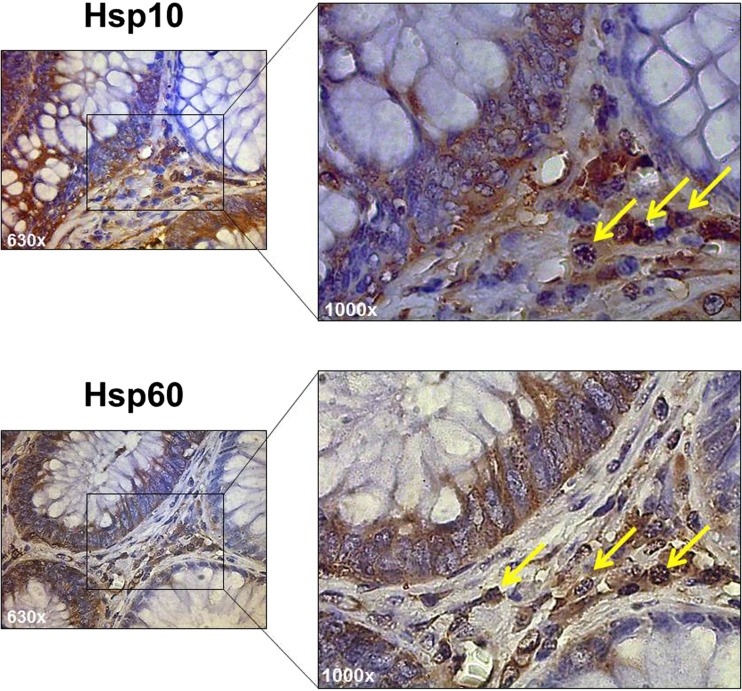
Fig. 3.
Tubular adenoma. Representative high-power images of immunostaining for Hsp10 and Hsp60 in lamina propria cells with the typical characteristics of inflammatory cells (arrows). (Magnification 630× and 1000×)
The data show (a) the numbers of Hsp60- and Hsp10-positive epithelial and lamina propria cells increase gradually throughout the carcinogenic steps from normal mucosa through tubular adenoma with moderate dysplasia to invasive adenocarcinoma; (b) the number of Hsp70-positive cells was significantly higher only in the epithelium of adenocarcinoma when compared to normal and dysplastic mucosa; no differences were detectable in the lamina propria; and (c) the number of Hsp90-positive cells was significantly higher only in the lamina propria of adenocarcinoma than in normal and dysplastic mucosa; no differences were detectable in the epithelium. No correlation was found between Hsps immunohistochemical levels and size and anatomical location of tubular adenomas or location and staging of adenocarcinomas.
Discussion
Adenocarcinoma was characterized by the highest levels of Hsp10 and Hsp60 in epithelium and lamina propria, accompanied by the highest levels of Hsp70 only in epithelium and of Hsp90 only in lamina propria, by comparison with normal and tubular adenoma counterparts.
On the other hand, a significant elevation of Hsp10 and Hsp60 only, i.e., in the absence of elevation of Hsp70 or Hsp90, in both mucosa layers was indicative of tubular adenoma. The results pertaining to Hsp10 and Hsp60, which are the first to show high levels from the beginning of the carcinogenic process and remain high until the last step, adenocarcinoma, suggest that these two chaperonins are implicated in cancer initiation and/or progression from the very early stages. Many reports support the idea that Hsps are implicated in the pathogenesis and in the progression of various human neoplasms (Ciocca and Calderwood 2005), but the mechanisms are not yet fully understood. Although Hsp60 and Hsp10 perform their canonical “chaperoning” functions in both prokaryotic and eukaryotic cells (Macario and Conway de Macario 2005), they have also acquired, probably during evolution, “extra-chaperoning” roles. Among these roles are some pertaining to the mechanisms of carcinogenesis in a variety of cancer types (Czarnecka et al. 2006a). Hsp60 and Hsp10 may play a role in promoting the growth and proliferation of cancer cells by protecting them from apoptosis. Although Hsp60 is a molecule with specific functions related to mitochondrial protein folding, working together with its co-chaperone Hsp10 (Czarnecka et al. 2006a), the chaperonin may interact with molecules that participate in the process of apoptosis. For example, Hsp60 can stimulate anti-apoptotic mechanisms involving sequestration of Bax-containing complexes, survivin, and p53, thus favoring tumor cell survival (Ghosh et al. 2008; Gupta and Knowlton 2002; Shan et al. 2003).
In cancer cells, Hsp60 is localized not only in and around the mitochondria as in normal cells but also in the cytosol very close to the plasma membrane and in the plasma membrane (Campanella et al. 2015). Hsp60seems to transit from the cell to the peritumoral environment and, in this context, might bind to the receptors present on the surface of inflammatory cells (such as macrophages and NK cells), leading to secretion of cytokines essential for immune surveillance. There are reports that emphasize the role of Hsp60 as a ligand of toll-like receptor-4 (TLR-4) and, consequently, as activator of innate and adaptive immunities (Gupta and Knowlton 2007; Ohashi et al. 2000). The activation of TLR-4 has a key role in regulation of T cell- and B cell-mediated immune responses (Kapsenberg 2003; Pasare and Medzhitov 2005) but also participates in the activation of the NF-κB pathway, which is known to link chronic inflammation and tumor development (Chow et al. 2012). The peritumoral microenvironment contains innate immune cells (macrophages, dendritic cells, natural killer cells) and adaptive immune cells (T and B lymphocytes) that communicate with each other by means of direct contact or cytokine and chemokine production and act to control and shape tumor growth. The same cancer cells constantly edit and modulate the host anti-tumor immune response, and the host immune response shapes tumor immunogenicity and clonal selection. During this process, the balance between anti-tumor and tumor-promoting immunity can be tilted in favor of tumor growth (Grivennikov et al. 2010). Tumors can induce the recruitment of regulatory T cells and myeloid derived suppressor cells, both that are regulatory immune cells capable of inhibiting the host-protective anti-tumor response (Ostrand-Rosenberg and Sinha 2009; Zou et al. 2006). Thus, in addition to the tumor-suppressor function represented by the elimination of nascent transformed tumor cells (cancer immune surveillance), the tumor microenvironment can also select immune cells that are able to facilitate tumor growth.
These concepts ought to encourage research for elucidating at the molecular and mechanistic levels the role of Hsp10 and Hsp60 in carcinogenesis and, eventually, developing therapeutic means targeting one or both chaperonins.
In this work, we have studied the levels of Hsp10, Hsp60, Hsp70, and Hsp90 by immunohistochemistry in biopsies of human mucosa of large bowel taken from normal control subjects (normal mucosa), pre-neoplastic lesions (tubular adenoma), and invasive neoplasms (adenocarcinoma). The main purpose was to identify markers in the adenoma that would indicate the future of the lesion, namely if it would proceed to carcinoma or not. The results show that Hsp10 and Hsp60 levels are higher in pre-neoplastic lesions as well as in cancer lesions compared to normal mucosa. By contrast, Hsp70 and Hsp90 levels were increased only in the final stages, i.e., adenocarcinoma, while there was no difference between normal and pre-neoplastic lesions for these two chaperones. Hsp70 levels were higher only in the epithelial cells of the cancerous tissue than in the tubular adenomas and normal mucosa. Hsp90 levels were higher only in the lamina propria of the tumoral tissue than in tubular adenoma and normal mucosa. In conclusion, only Hsp60 and Hsp10 levels were found to characterize the adenoma lesions and be also present at high levels in the fully developed adenocarcinoma. Thus, the presence of elevated levels of the two chaperonins in adenoma is an indicator of bad prognosis and should help the pathologist make adequate predictions of disease progression.
Several articles have been published about the Hsps and cancer (see for example Lianos et al. 2015). High levels of Hsp10 in the cytoplasm of cancer cells have been reported in the past (Cappello et al. 2005c). Also, Hsp60 has been shown to be augmented and localized to the cytoplasm of cancer cells and its implication in the pathogenesis of a range of human cancer types has been proposed (Cappello et al. 2005c; Rappa et al. 2012; Campanella et al. 2015). For example, it has been reported that Hsp60 promotes tumor cell growth in breast, lung, and colon cancer, by stabilizing the levels of survivin and overcoming the apoptotic stimuli (Ghosh et al. 2008). In another study, the levels of Hsp60 in several cases of gastric cancer were found elevated and were closely associated with tumor aggressiveness (Li et al. 2014). In contrast, other authors have shown lower levels of Hsp60 in various types of human cancer (Ito et al. 1998; Lebret et al. 2003; Cappello et al. 2005a, 2006). Hsp70 and Hsp90 have also been found increased in several types of cancer in which they may promote the carcinogenic process by inhibiting apoptosis (Beere 2001) and by binding tumor-suppressor proteins, such as p53 and HER2 (Vargas-Roig et al. 1998).
The immunohistochemical study reported here is, to our knowledge, the first in which a comparative evaluation of Hsp10, Hsp60, Hsp70, and Hsp90 levels was carried out with biopsies from tubular adenoma cases taken by us as examples of large bowel pre-neoplastic lesions. In the literature, there are several studies on cancer and Hsps in which comparisons were made but only between normal and neoplastic tissues. Our results show that among the four Hsps studied, only Hsp10 and Hsp60 levels are higher in both epithelial and lamina propria layers of pre-neoplastic as well as in cancer lesions compared to normal mucosa. Therefore, we hypothesize that these molecules are involved in the very early steps of carcinogenesis in the large bowel. For this reason, Hsp10 and Hsp60 are not only promising candidates as diagnostic biomarkers, but they should also be taken into consideration in the design of therapeutic means aiming at the chaperones.
Acknowledgments
A.J.L.M. and E.C. de M. were partially supported by IMET; A.J.L.M. and F.C. were partially supported by IEMEST. This work was done under the umbrella of the agreement between the Euro-Mediterranean Institute of Science and Technology (IEMEST, Italy) and the Institute of Marine and Environmental Technology (IMET, USA) signed in March 2012 (this is IMET contribution number IMET 16-176).
Compliance with ethical standards
Founding sources
The work was partially supported by the Euro-Mediterranean Institute of Science and Technology (IEMEST) and the University of Palermo. In this work were used instruments provided by IEMEST and funded by the Italian National Operational Programme for Research and Competitiveness 2007–2013 grant (Project code: PONa3_00210, European Regional Development Fund).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Francesca Rappa and Alessandro Pitruzzella contributed equally to the present work.
References
- Beere HM (2001) Stressed to death: regulation of apoptotic signaling pathways by the heat-shock proteins. Sci STKE: re1 [DOI] [PubMed]
- Calderwood SK, Theriault JR, Gong J. Message in a bottle: role of the 70-kDa heat-shock protein family in antitumor immunity. Eur J Immunol. 2005;35:2518–2527. doi: 10.1002/eji.200535002. [DOI] [PubMed] [Google Scholar]
- Campanella C, Rappa F, Sciumè C, Marino Gammazza A, Barone R, Bucchieri F, et al. Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer. 2015;121:3230–3239. doi: 10.1002/cncr.29499. [DOI] [PubMed] [Google Scholar]
- Cappello F, Di Stefano A, D'Anna SE, Donner CF, Zummo G. Immunopositivity of heat shock protein 60 as a biomarker of bronchial carcinogenesis. Lancet Oncol. 2005;6:816. doi: 10.1016/S1470-2045(05)70393-4. [DOI] [PubMed] [Google Scholar]
- Cappello F, Zummo G. HSP60 expression during carcinogenesis: a molecular "proteus" of carcinogenesis? Cell Stress Chaperones. 2005;10:263–264. doi: 10.1379/1466-1268(2005)10[263:HEDCAM]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello F, David S, Rappa F, Bucchieri F, Marasà L, Bartolotta TE, et al. The expression of HSP60 and HSP10 in large bowel carcinomas with lymph node metastase. BMC Cancer. 2005;5:139. doi: 10.1186/1471-2407-5-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello F, Di Stefano A, David S, Rappa F, Anzalone R, La Rocca G, et al. Hsp60 and Hsp10 down-regulation predicts bronchial epithelial carcinogenesis in smokers with chronic obstructive pulmonary disease. Cancer. 2006;107:2417–2424. doi: 10.1002/cncr.22265. [DOI] [PubMed] [Google Scholar]
- Chow MT, Möller A, Smyth MJ. Inflammation and immune surveillance in cancer. Semin Cancer Biol. 2012;22:23–32. doi: 10.1016/j.semcancer.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Czarnecka AM, Campanella C, Zummo G, Cappello F. Mitochondrial chaperones in cancer: from molecular biology to clinical diagnostics. Cancer Biol Ther. 2006;5:714–720. doi: 10.4161/cbt.5.7.2975. [DOI] [PubMed] [Google Scholar]
- Czarnecka AM, Campanella C, Zummo G, Cappello F. Heat shock protein 10 and signal transduction: a "capsula eburnea" of carcinogenesis? Cell Stress Chaperones. 2006;11:287–294. doi: 10.1379/CSC-200.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Felice V, Ardizzone N, Marcianò V, Bartolotta T, Cappello F, Farina F, et al. Senescence-associated HSP60 expression in normal human skin fibroblasts. Anat Rec A Discov Mol Cell Evol Biol. 2005;284:446–453. doi: 10.1002/ar.a.20181. [DOI] [PubMed] [Google Scholar]
- Ghosh JC, Dohi T, Kang BH, Altieri DC. Hsp60 regulation of tumor cell apoptosis. J Biol Chem. 2008;283:5188–5194. doi: 10.1074/jbc.M705904200. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Knowlton AA. Cytosolic heat shock protein 60, hypoxia, and apoptosis. Circulation. 2002;106:2727–2733. doi: 10.1161/01.CIR.0000038112.64503.6E. [DOI] [PubMed] [Google Scholar]
- Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol. 2007;292:H3052–H3056. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- Ito T, Kawabe R, Kurasono Y, Hara M, Kitamura H, Fujita, et al. Expression of heat shock proteins in squamous cell carcinoma of the tongue: an immunohistochemical study. J Oral Pathol Med. 1998;27:18–22. doi: 10.1111/j.1600-0714.1998.tb02085.x. [DOI] [PubMed] [Google Scholar]
- Joly AL, Wettstein G, Mignot G, Ghiringhelli F, Garrido C. Dual role of heat-shock proteins as regulator of apoptosis and innate immunity. J Innate Immun. 2010;2:238–247. doi: 10.1159/000296508. [DOI] [PubMed] [Google Scholar]
- Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- Kirchhoff SR, Gupta S, Knowlton AA. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation. 2002;105:2899–2904. doi: 10.1161/01.CIR.0000019403.35847.23. [DOI] [PubMed] [Google Scholar]
- Lebret T, Watson RW, Molinié V, O’Neill A, Gabriel C, Fitzpatrick JM, et al. Heat-shock proteins HSP27, HSP60, HSP70, and HSP90: expression in bladder carcinoma. Cancer. 2003;98:970–977. doi: 10.1002/cncr.11594. [DOI] [PubMed] [Google Scholar]
- Li XS, Xu Q, Fu XY, Luo WS. Heat shock protein 60 overexpression is associated with the progression and prognosis in gastric cancer. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lianos GD, Alexiou GA, Mangano A, Mangano A, Rausei S, Boni L, et al. The role of heat shock proteins in cancer. Cancer Lett. 2015;360:114–118. doi: 10.1016/j.canlet.2015.02.026. [DOI] [PubMed] [Google Scholar]
- Macario AJL, Conway de Macario E. Sick chaperones, cellular stress and disease. N Engl J Med. 2005;353:1489–1501. doi: 10.1056/NEJMra050111. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Control of B-cell responses by toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Muthana M, Calderwood SK. The dual immunoregulatory roles of stress proteins. Trends Biochem Sci. 2008;33:71–79. doi: 10.1016/j.tibs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Rappa F, Farina F, Zummo G, David S, Campanella C, Carini F, et al. HSP-molecular chaperones in cancer biogenesis and tumor therapy: an overview. Anticancer Res. 2012;32:5139–5150. [PubMed] [Google Scholar]
- Rappa F, Sciume C, Lo Bello M, Bavisotto CC, Marino Gammazza A, Barone R, et al. Comparative analysis of Hsp10 and Hsp90 expression in healthy mucosa and adenocarcinoma of the large bowel. Anticancer Res. 2014;34:4153–4159. [PubMed] [Google Scholar]
- Sanderson S, Valenti M, Gowan S, Patterson L, Ahmad Z, Workman P, et al. Benzoquinone ansamycin heat-shock protein 90 inhibitors modulate multiple functions required for tumor angiogenesis. Mol Cancer Ther. 2006;5:522–532. doi: 10.1158/1535-7163.MCT-05-0439. [DOI] [PubMed] [Google Scholar]
- Shan YX, Liu TJ, Su HF, Samsamshariat A, Mestril R, Wang PH. Hsp10 and Hsp60 modulate Bcl-2 family and mitochondria apoptosis signaling induced by doxorubicin in cardiac muscle cells. J Mol Cell Cardiol. 2003;35:1135–1143. doi: 10.1016/S0022-2828(03)00229-3. [DOI] [PubMed] [Google Scholar]
- Vargas-Roig LM, Gaqo FE, Tello O, Aznar JC, Ciocca DR. Heat-shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer. 1998;79:468–475. doi: 10.1002/(SICI)1097-0215(19981023)79:5<468::AID-IJC4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Voellmy R. Transduction of the stress signal and mechanisms of transcriptional regulation of heat shock/stress protein expression in higher eukaryotes. Crit Rev Eukaryot Gene Expr. 1994;4:357–401. [PubMed] [Google Scholar]
- Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Walsh D, Grantham J, Zhu XO, Wei Lin J, van Oosterum M, Taylor R, et al. The role of heat shock proteins in mammalian differentiation and development. Environ Med. 1999;43:79–87. [PubMed] [Google Scholar]
- Zhao L, Liu L, Wang S, Zhang YF, Yu L, Ding YQ. Differential proteomic analysis of human colorectal carcinoma cell lines metastasis-associated proteins. J Cancer Res Clin Oncol. 2007;133:771–782. doi: 10.1007/s00432-007-0222-0. [DOI] [PubMed] [Google Scholar]
- Zou W, Regulatory T, cells, tumour immunity and immunotherapy Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]