FIGURE 1.
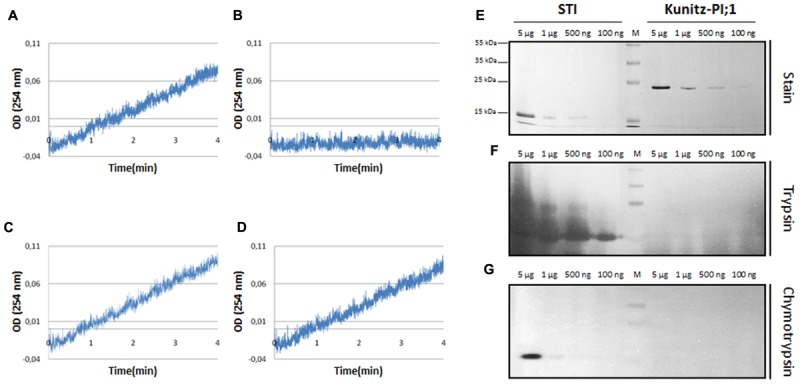
Analysis of protease-inhibitor activity of bacterially expressed and purified Arabidopsis Kunitz-PI;1. (A–D) Trypsin activity measured in the absence of protease inhibitors (A) or presence of either soybean trypsin inhibitor (B) or Arabidopsis Kunitz-PI;1 added at molar ratios of 1:1 (C) or 1:10 (D). Trypsin activity was determined by cleavage of BAEE and measuring the absorption of the product at 253 nm. (E–G) Reverse zymography to reveal protease inhibitor activity of soybean trypsin inhibitor (STI, left part) and Arabidopsis Kunitz-PI;1 (right part). (E) PIs were separated on a SDS-PAA gel and stained with Coomassie brilliant blue. (F as E) But showing soybean trypsin inhibitor (STI) (left part) and Arabidopsis Kunitz-PI;1 (right part) on a SDS-PAA gel containing gelatine after renaturation and incubation with trypsin. (G as F) But depicting the results obtained with chymotrypsin.
