Abstract
Introduction
Ischemia-modified albumin (IMA), a novel ischemia marker, and mean platelet volume (MPV), a determinant of platelet activation, have been reported as elevated markers in cardiovascular risk factors such as atherosclerosis, metabolic syndrome, diabetes mellitus (DM), hypertension, and dyslipidemia. As psoriasis is a chronic inflammatory disease having comorbidities, IMA and MPV can help determine the risk factors for psoriasis.
Aim
To investigate the correlation between the psoriasis area severity index (PASI), IMA and MPV levels in patients with psoriasis.
Material and methods
This cross-sectional, case-control study was performed between January 2014 and December 2014 at the University hospital in Çanakkale, Turkey. Forty-five patients with psoriasis and 44 healthy volunteers over 18 years of age were included in the study. In the psoriasis patient group, clinical features and PASI scores were recorded. Serum IMA and MPV concentrations were evaluated in both groups.
Results
The mean IMA values were 0.85 ±0.15 and 0.79 ±0.09 (in the psoriasis patients and control groups, respectively), and there was a statistically significant difference (p = 0.048). Ischemia-modified albumin levels were not correlated with PASI scores (r = 0.024; p = 0.889) but were correlated with disease duration (r = 0.323; p = 0.048). There was no statistically significant difference between the MPV values of the two groups (8.98 ±1.14 and 9.19 ±1.28 in the psoriasis patients and control groups, respectively) (p = 0.435).
Conclusions
Ischemia-modified albumin may be used as a marker for detecting oxidative stress in patients with psoriasis, especially those with a long disease duration.
Keywords: psoriasis, ischemia-modified albumin, mean platelet volume, psoriasis area severity index
Introduction
Psoriasis is an immune-mediated chronic disease involving primarily the skin. Today, psoriasis is defined as a multisystemic disorder. The psoriatic patient has several comorbidities such as diabetes, hypertension, and lipid abnormalities. There is an increased risk of metabolic syndrome and cardiovascular disorders in patients with psoriasis [1–3].
The amino terminal end (N-terminus) of albumin is the metal binding site of the molecule. In ischemic conditions, the form of the N-terminus changes, and the metal-binding capacity decreases. Thus, this new form is called ischemia-modified albumin (IMA), a novel ischemia marker [4–6]. In recent years, elevated levels of IMA are thought to be associated with several diseases based on oxidative stress [5–7]. The cardiovascular risk factors and IMA are reported to increase in obesity [8, 9], metabolic syndrome [10], type 2 diabetes mellitus (DM) [11], and hypercholesterolemia [12].
Mean platelet volume (MPV) is a determinant of platelet activation. The volume increases in acute coronary syndrome and has been suggested as a new marker for early detection of cardiovascular risk factors such as atherosclerosis [13], metabolic syndrome [14], DM [14], arterial hypertension [15], and dyslipidemia [16].
Aim
Based on this information, we aimed to investigate the IMA and MPV levels in psoriatic patients to define the correlation between psoriasis and the risk of comorbidities by using IMA and MPV as early markers.
Material and methods
Design and setting
A case-control study was performed to define the correlation between the psoriasis area severity index and ischemia modified albumin, mean platelet volume levels. We performed the study at the dermatology department of Canakkale Onsekiz Mart University, Faculty of Medicine, in Turkey, between January 2014 and December 2015. The control group was recruited from patients without psoriasis who visited the dermatology department. Patients suffering from inflammatory skin conditions, autoimmune diseases, or any cardiovascular disease were excluded from the control group. Informed consent was obtained from all patients. Forty-five patients with psoriasis and 44 healthy volunteers over 18 years of age were included in the study. In the psoriasis patient group, clinical features and psoriasis area severity index (PASI) scores were recorded. The study was performed based on the Helsinki Declaration with approval of the Çanakkale Onsekiz Mart University Local Ethical Committee.
Laboratory analysis
Fasting blood samples were collected from all subjects by venous puncture technique in tubes with ethylenediaminetetraacetic acid (EDTA) to prevent coagulation for biochemical determinations. The blood samples were centrifuged to obtain serum for IMA procedure at 3,000 rpm for 10 min and all of them were stored at –80°C until analysis.
Ischemia-modified albumin measurement
The ischemia-modified albumin measurement was performed by a rapid colorimetric method depends on albumin cobalt binding that was developed by Bar-Or et al. [17]. Two hundred µl of patient serum and 50 µl of a solution of 1 g/l cobalt chloride (Sigma, CoCl2. 6 H2O) was mixed and shaken gently for a few seconds. The mixture was incubated for approximately 10 min to cobalt albumin binding. The amount of albumin-bound cobalt was visualized by adding dithiothreitol (DTT) (Sigma, 1.5 mg/ml H2O) as a colorizing agent. After a 2-min incubation at room temperature, 1.0 ml of 0.9% NaCl was added to stop the reaction. The amount of albumin-bound cobalt was measured spectrophotometrically at 470 nm (Hitachi U-2900 Spectrophotometer) in comparison with a serum cobalt blank without DTT. Finally, the intensity of the color formation cause a higher level of absorbance, thus the results were shown as absorbance units (ABSUs). The MPV measurements were performed with an electrical impedance method by Beckman Coulter LH 780 analyzer (Beckman Coulter, Inc., CA, USA).
Statistical analysis
Statistical analysis was done with the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA), version 19.0. Compliance with the normal distribution of the variables was analyzed with the Kolmogorov-Smirnov test. Data mean, standard deviation, frequency, and percentage values were used in the descriptive data presentation. The t test was used in the independent samples to compare the mean values for the psoriasis patient and control groups. P-values under 0.05 were considered statistically significant.
Results
A total of 45 patients with psoriasis and 44 healthy controls were included in this study. Twenty-two (48.9%) of the 45 patients with psoriasis were female, and 23 (51.1%) were male; 22 (50%) patients in the control group were female, and 22 (50%) were male. The mean age of the patients with psoriasis was 46.00 ±14.62, and that of the control group was 44.2 ±13.6. The demographic characteristics of the patients with psoriasis are summarized in Table 1.
Table 1.
Demographic characteristics of psoriatic patients
| Characteristics | Result Mean ± SD or n (%) |
|---|---|
| Age [years] | 46.00 ±14.62 |
| Gender: female | 22 (48.9) |
| Family history of present | 14 (31.8) |
| BMI [kg/m2]: | |
| < 18.5 | 0 (0) |
| 18.5–24.99 | 9 (22.0) |
| 25–30 | 14 (34.1) |
| > 30 | 18 (43.9) |
| Disease duration [years] | 13.7 ±11.3 |
| PASI | 16.3 ±11.2 |
BMI – body mass index.
At the end of the study, the mean MPV values were identified as 8.98 ±1.14 and 9.19 ±1.28 (in the psoriasis patient and control groups, respectively), and there was no statistically significant difference between the two groups (p = 0.435) (Table 2).
Table 2.
The comparison of IMA and MPV levels in patients with psoriasis and healthy controls
| Parameter | Patients (n = 45) Mean ± standard deviation |
Controls (n = 44) Mean ± standard deviation |
P-value |
|---|---|---|---|
| MPV | 8.98 ±1.14 | 9.19 ±1.28 | 0.435 |
| IMA | 0.85 ±0.15 | 0.79 ±0.09 | 0.048 |
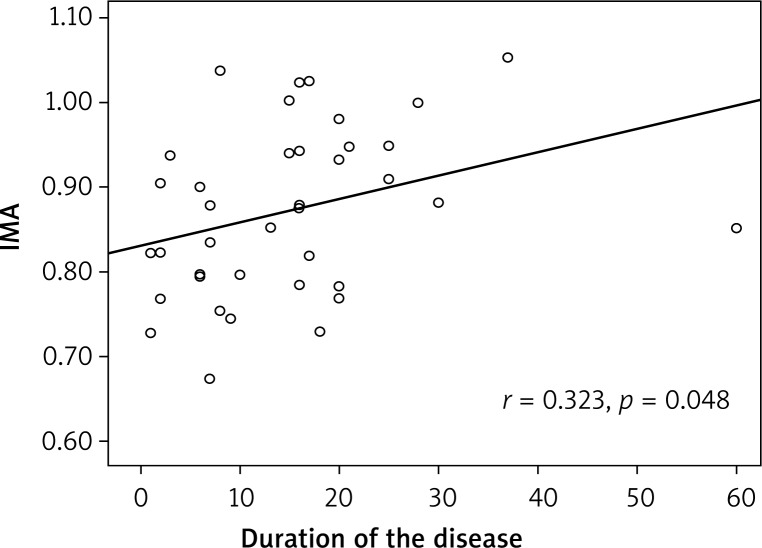
The mean IMA values were 0.85 ±0.15 and 0.79 ±0.09 (in the psoriasis patient and control groups, respectively), and there was a statistically significant difference (p = 0.048) (Table 2). There was no correlation between IMA levels and PASI scores (r = 0.024; p = 0.889, Table 3), yet a positive correlation between IMA levels and disease duration was observed (r = 0.323; p = 0.048) (Figure 1 and Table 3).
Table 3.
The correlation between IMA, PASI, MPV and disease duration
| Parameter | IMA | PASI | MPV | Disease duration [year] | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| IMA | – | – | 0.024 | 0.889 | 0.054 | 0.756 | 0.323 | 0.048 |
| PASI | 0.024 | 0.889 | – | – | 0.076 | 0.652 | – | – |
| MPV | 0.054 | 0.756 | –0.076 | 0.652 | – | – | 0.134 | 0.409 |
| Disease duration [year] | 0.323 | 0.048 | – | – | 0.134 | 0.409 | – | – |
*p < 0.05; Pearson correlation test.
Figure 1.
The positive correlation between serum ischemiamodified albumin (IMA) and duration of disease in patients with psoriasis
Discussion
Ischemia-modified albumin is accepted as a marker of myocardial ischemia by the U.S. Food and Drug Administration. In ischemic conditions, the metal-binding (cobalt, copper, zinc) capacity of albumin decreases as a result of exposure to reactive oxygen species. Ischemia-modified albumin is not specific for myocardial damage only. Patients with cancer, infections, end-stage renal disease, liver disease, brain ischemia, metabolic syndrome, type 2 DM, and hypercholesterolemia has increased IMA values [10–12, 18, 19]. Recently, IMA was also described as a marker for diseases related to inflammation [7–10]. Platelet activation has also been demonstrated in inflammatory diseases in addition to the platelets’ main role in hemostasis and thrombosis. Mean platelet volume is a marker indicating platelet activation and the risk of atherothrombosis [20].
Since psoriasis is a chronic and immune mediated skin disease, we analyzed MPV and IMA levels in psoriasis to determine the correlation between disease severity and the markers. We found a significant difference between the IMA levels of the psoriatic patients and the healthy controls (p < 0.05), but there was no significant difference between the MPV levels of the two groups. In addition, according to our findings, the IMA and MPV levels were not correlated with the PASI scores, yet the correlation between IMA levels and disease duration was statistically significant (p < 0.05). Ozdemir et al. first reported higher IMA levels in patients with psoriasis than in healthy controls [21]. However, the researchers reported has not reported any correlation between IMA levels and disease duration or PASI scores yet.
Similar pathogenetic mechanisms have been reported in the development of atherosclerosis and psoriasis. Cytokines such as tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and interleukin (IL)-2 and platelet activation increase in both cases [2]. Saleh et al. investigated platelet markers that may be associated with subclinical atherosclerosis in patients with psoriasis [22]. However, the researchers did not find a significant difference in the MPV values of patients with psoriasis. In contrast, Canpolat et al. reported increased MPV levels in patients with psoriasis and psoriatic arthritis [23]. In our study, we thought MPV could be valuable in determining the atherosclerotic risk factors for psoriasis. However, we did not find any significant difference in the MPV values of patients with psoriasis. This may be related to the number of patients.
Increased levels of markers of oxidative stress or decreased levels of antioxidant molecules have been reported in patients with psoriasis [24]. In our study, increased IMA levels in patients with psoriasis support the role of oxidative stress in the pathogenesis of psoriasis. Ischemia-modified albumin levels may be higher in patients with psoriasis because the increased oxygen radicals affect the structure of albumin.
The small sample size of the current study is its major limitation. Patients with serious cardiac problems such as myocardial infarction and heart failure were excluded from our study. Obesity, smoking, and alcohol, which may affect oxidative stress, were not excluded since they were common lifestyle factors in both groups. In addition, we compared patients with psoriasis with or without comorbidities, and there was no statistically significant difference between the two groups.
Conclusions
We suggest that IMA may be used as a marker for detecting oxidative stress and the risk of comorbidities of patients with psoriasis and those have long disease duration. Oxidative stress biomarkers such as IMA will lead to new therapeutic approaches to psoriasis. Large randomized controlled trials are needed to investigate these relations.
Acknowledgments
This study was conducted in the Dermatology Department of Training and Research Hospital of Canakkale Onsekiz Mart University.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Madanagobalane S, Anandan S. Prevalence of metabolic syndrome in south Indian patients with psoriasis vulgaris and the relation between disease severity and metabolic syndrome: a hospital-based case-control study. Indian J Dermatol. 2012;57:353–7. doi: 10.4103/0019-5154.100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Komorowska OR, Szczerkowska-Dobosz A, Purzycka-Bohdan D, et al. Psoriasis as a risk factor of cardiovascular diseases. Przegl Dermatol. 2014;101:500–6. [Google Scholar]
- 3.Owczarczyk-Saczonek AB, Nowicki RJ. Prevalence of cardiovascular disease risk factors, and metabolic syndrome and its components in patients with psoriasis aged 30 to 49 years. Postep Derm Alergol. 2015;32:290–5. doi: 10.5114/pdia.2014.40966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippi G, Montagnana M, Guidi GC. Albumin cobalt binding and ischemia modified albumin generation: an endogenous response to ischemia? Int J Cardiol. 2006;108:410–1. doi: 10.1016/j.ijcard.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 5.Ertekin B, Kocak S, Dundar D, et al. Diagnostic value of ischemia-modified albumin in acute coronary syndrome and acute ischemic stroke. Pak J Med Sci. 2013;29:1003–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Demir B, Ozyazgan S, Korkmaz GG, et al. The relationship between ischemia modified albumin and oxidative stress parameters in patients with cardiac syndrome X. Clin Lab. 2013;59:1319–29. doi: 10.7754/clin.lab.2013.121142. [DOI] [PubMed] [Google Scholar]
- 7.Kadioğlu H, Ömür D, Bozkurt S, et al. Ischemia modified albumin can predict necrosis at incarcerated hernias. Dis Markers. 2013;35:807–10. doi: 10.1155/2013/185425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baysal T, Alp H, Koç N, et al. Serum ischemia-modified albumin level and its association with cardiovascular risk factors in obese children and adolescents. J Pediatr Endocrinol Metab. 2012;25:935–44. doi: 10.1515/jpem-2012-0191. [DOI] [PubMed] [Google Scholar]
- 9.Mehmetoğlu I, Kurban S, Yerlikaya FH, et al. Obesity is an independent determinant of ischemia-modified albumin. Obes Facts. 2012;5:700–9. doi: 10.1159/000343954. [DOI] [PubMed] [Google Scholar]
- 10.Valle Gottlieb MG, da Cruz IB, Duarte MM, et al. Associations among metabolic syndrome, ischemia, inflammatory, oxidatives, and lipids biomarkers. J Clin Endocrinol Metab. 2010;95:586–91. doi: 10.1210/jc.2009-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piwowar A, Knapik-Kordecka M, Warwas M. Ischemia-modified albumin level in type 2 diabetes mellitus – preliminary report. Dis Markers. 2008;24:311–7. doi: 10.1155/2008/784313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duarte MM, Rocha JB, Moresco RN, et al. Association between ischemia-modified albumin, lipids and inflammation biomarkers in patients with hypercholesterolemia. Clin Biochem. 2009;42:666–71. doi: 10.1016/j.clinbiochem.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Gulcan AR, Karakaş MS, Akdemir B, et al. Relation between mean platelet volume and subclinical atherosclerosis in patients with metabolic syndrome. Turk Kardiyol Derm Ars. 2014;42:22–8. doi: 10.5543/tkda.2014.50708. [DOI] [PubMed] [Google Scholar]
- 14.Shah B, Sha D. The relationship between diabetes, metabolic syndrome, and platelet activity as measured by mean platelet volume: the National Health And Nutrition Examination Survey, 1999-2004. Diabetes Care. 2012;35:1074–8. doi: 10.2337/dc11-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inanc T, Kaya MG. The mean platelet volume in patients with non-dipper hypertension compared to dippers and normotensives. Blood Press. 2010;19:81–5. doi: 10.3109/08037050903516284. [DOI] [PubMed] [Google Scholar]
- 16.Korkmaz L, Korkmaz AA, Akyüz AR, et al. Association between mean platelet volume and coronary artery calcification in patients without overt cardiovascular disease: an observational study. Anadolu Kardiyol Derg. 2012;12:35–9. doi: 10.5152/akd.2012.007. [DOI] [PubMed] [Google Scholar]
- 17.Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt albumin binding and its potential as a marker for myocardial ischemia – a preliminary report. J Emerg Med. 2000;19:311–5. doi: 10.1016/s0736-4679(00)00255-9. [DOI] [PubMed] [Google Scholar]
- 18.Ellidag HY, Bulbuller N, Eren E, et al. Ischemia-modified albumin: could it be a new oxidative stress biomarker for colorectal carcinoma? Gut Liver. 2013;7:675–80. doi: 10.5009/gnl.2013.7.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demir S, Avşar MK, Karakaya Z, et al. Increased mean platelet volume is associated with coronary artery ectasia. Postep Kardiol Inter. 2013;9:241–5. doi: 10.5114/pwki.2013.37502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamagawa-Mineoka R, Katoh N, Kishimoto S. Platelet activation in patients with psoriasis: increased plasma levels of platelet-derived microparticles and soluble P-selectin. J Am Acad Dermatol. 2010;62:621–6. doi: 10.1016/j.jaad.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 21.Ozdemir M, Kivici A, Balevi A, et al. Assessment of ischaemia-modified albumin level in patients with psoriasis. Clin Exp Dermatol. 2012;37:610–4. doi: 10.1111/j.1365-2230.2012.04384.x. [DOI] [PubMed] [Google Scholar]
- 22.Saleh HM, Attia EA, Onsy AM, et al. Platelet activation: a link between psoriasis per se and subclinical atherosclerosis a case-control study. Br J Dermatol. 2013;169:68–75. doi: 10.1111/bjd.12285. [DOI] [PubMed] [Google Scholar]
- 23.Canpolat F, Akpınar H, Eskioglu F. Mean platelet volume in psoriasis and psoriatic arthritis. Clin Rheumatol. 2010;29:325–8. doi: 10.1007/s10067-009-1323-8. [DOI] [PubMed] [Google Scholar]
- 24.Bacchetti T, Campanati A, Ferretti G, et al. Oxidative stress and psoriasis: the effect of antitumour necrosis factor-alpha inhibitor treatment. Br J Dermatol. 2013;168:984–9. doi: 10.1111/bjd.12144. [DOI] [PubMed] [Google Scholar]