Abstract
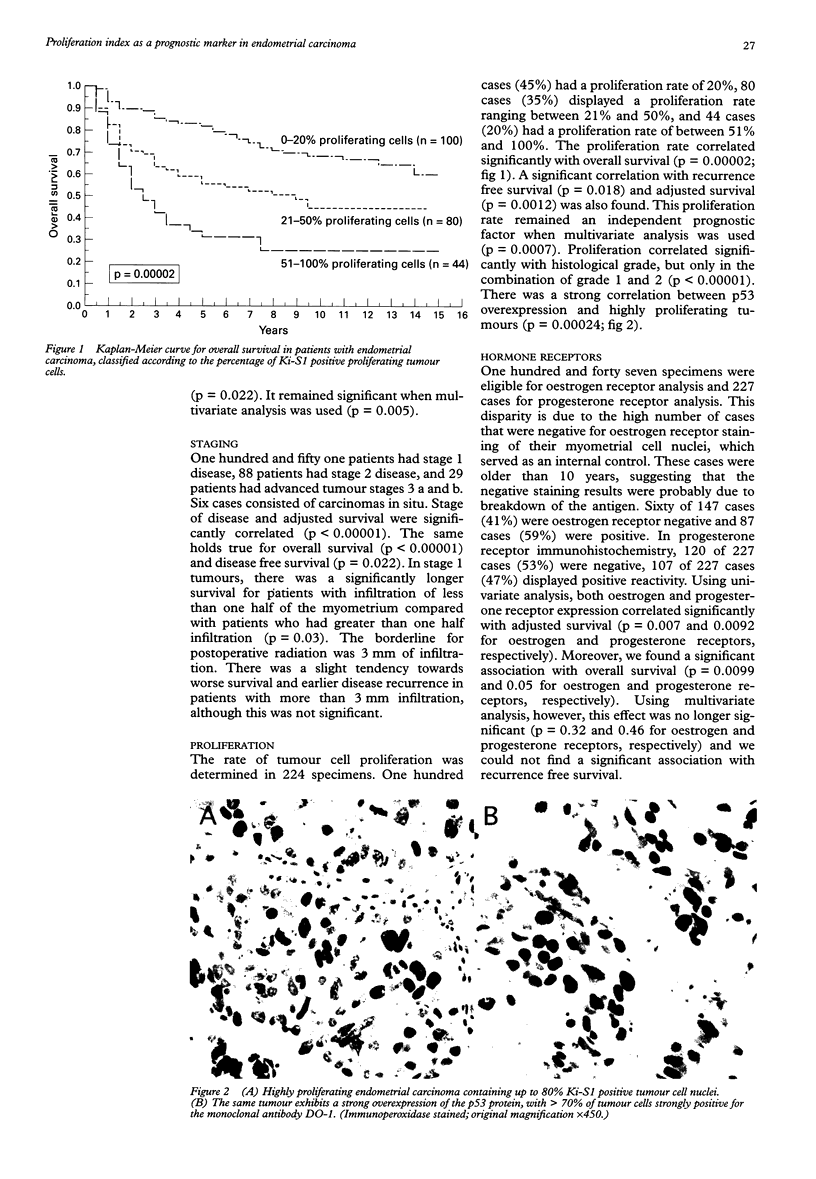
AIM: To test which immunohistochemically detected tumour parameters are predictive of outcome in endometrial carcinoma. METHODS: A retrospective study of 300 patients diagnosed with endometrial carcinoma between 1980 and 1985, ensuring a follow up of at least 10 years. Paraffin wax embedded tissues from 236 patients with endometrial carcinoma were evaluated in terms of histological tumour type and grade, stage of disease, and certain immunohistochemical biological parameters. These parameters included the expression of oestrogen and progesterone receptors, the expression of p53 protein, the expression of the c-erbB-2 oncoprotein, and the expression of protease cathepsin D, together with the rate of cell proliferation. RESULTS: Using univariate analysis, the following parameters correlated significantly with adjusted survival: histological type (p = 0.025), grade (p = 0.00003), FIGO stage (p < 0.00001), proliferation rate (p = 0.00002), oestrogen receptor expression (p = 0.007), progesterone receptor expression (p = 0.0092), and p53 expression (p = 0.00028). These parameters also correlated significantly with both disease free and overall survival. There was a weak correlation of cathepsin D expression with survival, but no correlation of c-erb B-2 expression with survival. Using multivariate analysis, only FIGO stage (p = 0.0021), histological grade (p = 0.005), and proliferation rate (p = 0.0007) remained statistically significant prognosticators of adjusted survival as well as of disease free and overall survival. CONCLUSIONS: In addition to conventional histological parameters, the immunohistochemical determination of proliferative activity could contribute to the identification of a high risk subgroup of endometrial carcinomas. The other parameters tested were not of significant additional predictive value.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambros R. A., Vigna P. A., Figge J., Kallakury B. V., Mastrangelo A., Eastman A. Y., Malfetano J., Figge H. L., Ross J. S. Observations on tumor and metastatic suppressor gene status in endometrial carcinoma with particular emphasis on p53. Cancer. 1994 Mar 15;73(6):1686–1692. doi: 10.1002/1097-0142(19940315)73:6<1686::aid-cncr2820730622>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Berchuck A., Kohler M. F., Marks J. R., Wiseman R., Boyd J., Bast R. C., Jr The p53 tumor suppressor gene frequently is altered in gynecologic cancers. Am J Obstet Gynecol. 1994 Jan;170(1 Pt 1):246–252. doi: 10.1016/s0002-9378(94)70414-7. [DOI] [PubMed] [Google Scholar]
- Bigsby R. M., Li A. X., Bomalaski J., Stehman F. B., Look K. Y., Sutton G. P. Immunohistochemical study of HER-2/neu, epidermal growth factor receptor, and steroid receptor expression in normal and malignant endometrium. Obstet Gynecol. 1992 Jan;79(1):95–100. [PubMed] [Google Scholar]
- Christopherson W. M., Connelly P. J., Alberhasky R. C. Carcinoma of the endometrium. V. An analysis of prognosticators in patients with favorable subtypes and Stage I disease. Cancer. 1983 May 1;51(9):1705–1709. doi: 10.1002/1097-0142(19830501)51:9<1705::aid-cncr2820510924>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Creasman W. T. Adenocarcinoma of the uterine corpus. Curr Opin Obstet Gynecol. 1993 Feb;5(1):80–83. [PubMed] [Google Scholar]
- Creasman W. T., Morrow C. P., Bundy B. N., Homesley H. D., Graham J. E., Heller P. B. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987 Oct 15;60(8 Suppl):2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Creasman W. T. Prognostic significance of hormone receptors in endometrial cancer. Cancer. 1993 Feb 15;71(4 Suppl):1467–1470. doi: 10.1002/cncr.2820710411. [DOI] [PubMed] [Google Scholar]
- Dallenbach-Hellweg G., Lang-Avérous G., Hahn U. The value of immunohistochemistry in the differential diagnosis of endometrial carcinomas. APMIS Suppl. 1991;23:91–99. [PubMed] [Google Scholar]
- Garzetti G. G., Ciavattini A., Goteri G., De Nictolis M., Romanini C. Proliferating cell nuclear antigen in endometrial carcinoma: pretreatment identification of high-risk patients. Gynecol Oncol. 1996 Apr;61(1):16–21. doi: 10.1006/gyno.1996.0089. [DOI] [PubMed] [Google Scholar]
- Geisler J. P., Wiemann M. C., Zhou Z., Miller G. A., Geisler H. E. Proliferation index determined by MIB-1 and recurrence in endometrial cancer. Gynecol Oncol. 1996 Jun;61(3):373–377. doi: 10.1006/gyno.1996.0159. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Göhring U. J., Scharl A., Thelen U., Ahr A., Crombach G., Titius B. R. Prognostic value of cathepsin D in breast cancer: comparison of immunohistochemical and immunoradiometric detection methods. J Clin Pathol. 1996 Jan;49(1):57–64. doi: 10.1136/jcp.49.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel N. W., Sebo T. J., Wilson T. O., Keeney G. L., Roche P. C., Suman V. J., Hu T. C., Podratz K. C. Prognostic value of p53 and proliferating cell nuclear antigen expression in endometrial carcinoma. Gynecol Oncol. 1996 Aug;62(2):192–198. doi: 10.1006/gyno.1996.0214. [DOI] [PubMed] [Google Scholar]
- Inoue M., Okayama A., Fujita M., Enomoto T., Sakata M., Tanizawa O., Ueshima H. Clinicopathological characteristics of p53 overexpression in endometrial cancers. Int J Cancer. 1994 Jul 1;58(1):14–19. doi: 10.1002/ijc.2910580104. [DOI] [PubMed] [Google Scholar]
- Kadar N., Malfetano J. H., Homesley H. D. Determinants of survival of surgically staged patients with endometrial carcinoma histologically confined to the uterus: implications for therapy. Obstet Gynecol. 1992 Oct;80(4):655–659. [PubMed] [Google Scholar]
- Khalifa M. A., Mannel R. S., Haraway S. D., Walker J., Min K. W. Expression of EGFR, HER-2/neu, P53, and PCNA in endometrioid, serous papillary, and clear cell endometrial adenocarcinomas. Gynecol Oncol. 1994 Apr;53(1):84–92. doi: 10.1006/gyno.1994.1092. [DOI] [PubMed] [Google Scholar]
- Kohlberger P., Loesch A., Koelbl H., Breitenecker G., Kainz C., Gitsch G. Prognostic value of immunohistochemically detected HER-2/neu oncoprotein in endometrial cancer. Cancer Lett. 1996 Jan 2;98(2):151–155. [PubMed] [Google Scholar]
- Kohler M. F., Berchuck A., Davidoff A. M., Humphrey P. A., Dodge R. K., Iglehart J. D., Soper J. T., Clarke-Pearson D. L., Bast R. C., Jr, Marks J. R. Overexpression and mutation of p53 in endometrial carcinoma. Cancer Res. 1992 Mar 15;52(6):1622–1627. [PubMed] [Google Scholar]
- Kreipe H., Alm P., Olsson H., Hauberg M., Fischer L., Parwaresch R. Prognostic significance of a formalin-resistant nuclear proliferation antigen in mammary carcinomas as determined by the monoclonal antibody Ki-S1. Am J Pathol. 1993 Feb;142(2):651–657. [PMC free article] [PubMed] [Google Scholar]
- Mäentausta O., Boman K., Isomaa V., Stendahl U., Bäckström T., Vihko R. Immunohistochemical study of the human 17 beta-hydroxysteroid dehydrogenase and steroid receptors in endometrial adenocarcinoma. Cancer. 1992 Sep 15;70(6):1551–1555. doi: 10.1002/1097-0142(19920915)70:6<1551::aid-cncr2820700618>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Nazeer T., Church K., Amato C., Ambros R. A., Rosano T. G., Malfetano J. H., Ross J. S. Comparative quantitative immunohistochemical and immunoradiometric determinations of cathepsin D in endometrial adenocarcinoma: predictors of tumor aggressiveness. Mod Pathol. 1994 May;7(4):469–474. [PubMed] [Google Scholar]
- Nazeer T., Malfetano J. H., Rosano T. G., Ross J. S. Correlation of tumor cytosol cathepsin D with differentiation and invasiveness of endometrial adenocarcinoma. Am J Clin Pathol. 1992 Jun;97(6):764–769. doi: 10.1093/ajcp/97.6.764. [DOI] [PubMed] [Google Scholar]
- Nielsen A. L., Nyholm H. C. Proliferative activity as revealed by Ki-67 in uterine adenocarcinoma of endometrioid type: comparison of tumours from patients with and without previous oestrogen therapy. J Pathol. 1993 Nov;171(3):199–205. doi: 10.1002/path.1711710308. [DOI] [PubMed] [Google Scholar]
- Pezzella F., Micklem K., Turley H., Pulford K., Jones M., Kocialkowski S., Delia D., Aiello A., Bicknell R., Smith K. Antibody for detecting p53 protein by immunohistochemistry in normal tissues. J Clin Pathol. 1994 Jul;47(7):592–596. doi: 10.1136/jcp.47.7.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A. L., Barbuto D. A., Chen D., Ramos L., Lagasse L. D., Karlan B. Y. HER-2/neu, p53, and DNA analyses as prognosticators for survival in endometrial carcinoma. Obstet Gynecol. 1995 May;85(5 Pt 1):729–734. doi: 10.1016/0029-7844(95)00037-r. [DOI] [PubMed] [Google Scholar]
- Poremba C., Bankfalvi A., Dockhorn-Dworniczak B. Das Tumorsuppressorgen p53. Die theoretischen Grundlagen und ihre Bedeutung für die Pathologie. Pathologe. 1996 May;17(3):181–188. doi: 10.1007/s002920050153. [DOI] [PubMed] [Google Scholar]
- Porter P. L., Gown A. M., Kramp S. G., Coltrera M. D. Widespread p53 overexpression in human malignant tumors. An immunohistochemical study using methacarn-fixed, embedded tissue. Am J Pathol. 1992 Jan;140(1):145–153. [PMC free article] [PubMed] [Google Scholar]
- Remmele W., Stegner H. E. Vorschlag zur einheitlichen Definition eines Immunreaktiven Score (IRS) für den immunhistochemischen Ostrogenrezeptor-Nachweis (ER-ICA) im Mammakarzinomgewebe. Pathologe. 1987 May;8(3):138–140. [PubMed] [Google Scholar]
- Saffari B., Jones L. A., el-Naggar A., Felix J. C., George J., Press M. F. Amplification and overexpression of HER-2/neu (c-erbB2) in endometrial cancers: correlation with overall survival. Cancer Res. 1995 Dec 1;55(23):5693–5698. [PubMed] [Google Scholar]
- Schink J. C., Rademaker A. W., Miller D. S., Lurain J. R. Tumor size in endometrial cancer. Cancer. 1991 Jun 1;67(11):2791–2794. doi: 10.1002/1097-0142(19910601)67:11<2791::aid-cncr2820671113>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Segreti E. M., Novotny D. B., Soper J. T., Mutch D. G., Creasman W. T., McCarty K. S. Endometrial cancer: histologic correlates of immunohistochemical localization of progesterone receptor and estrogen receptor. Obstet Gynecol. 1989 May;73(5 Pt 1):780–785. [PubMed] [Google Scholar]
- Sozzi G., Miozzo M., Donghi R., Pilotti S., Cariani C. T., Pastorino U., Della Porta G., Pierotti M. A. Deletions of 17p and p53 mutations in preneoplastic lesions of the lung. Cancer Res. 1992 Nov 1;52(21):6079–6082. [PubMed] [Google Scholar]
- Wang D., Konishi I., Koshiyama M., Mandai M., Nanbu Y., Ishikawa Y., Mori T., Fujii S. Expression of c-erbB-2 protein and epidermal growth receptor in endometrial carcinomas. Correlation with clinicopathologic and sex steroid receptor status. Cancer. 1993 Nov 1;72(9):2628–2637. doi: 10.1002/1097-0142(19931101)72:9<2628::aid-cncr2820720918>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Wolfson A. H., Sightler S. E., Markoe A. M., Schwade J. G., Averette H. E., Ganjei P., Hilsenbeck S. G. The prognostic significance of surgical staging for carcinoma of the endometrium. Gynecol Oncol. 1992 May;45(2):142–146. doi: 10.1016/0090-8258(92)90276-o. [DOI] [PubMed] [Google Scholar]
- Yabushita H., Masuda T., Sawaguchi K., Noguchi M., Nakanishi M. Growth potential of endometrial cancers assessed by a Ki-67 Ag/DNA dual-color flow-cytometric assay. Gynecol Oncol. 1992 Mar;44(3):263–267. doi: 10.1016/0090-8258(92)90054-m. [DOI] [PubMed] [Google Scholar]



