Abstract
Standard treatment for Glanzmann thrombasthenia is platelet transfusion. Recombinant activated factor VII has been shown to be successful in patients with Glanzmann thrombasthenia with platelet antibodies or who are refractory to platelet transfusions. The Glanzmann Thrombasthenia Registry prospectively collected worldwide information on the effectiveness and safety of platelet transfusion, recombinant activated factor VII and/or antifibrinolytics for the treatment of bleeds in patients with Glanzmann thrombasthenia. Data relating to 829 non-surgical bleeding episodes were entered into the Glanzmann Thrombasthenia Registry (severe/moderate: 216/613; spontaneous/post-traumatic: 630/199). Recombinant activated factor VII alone was used in 124/829 bleeds, recombinant activated factor VII+antifibrinolytics in 107/829, platelets±antifibrinolytics in 312/829, antifibrinolytics alone in 219/829, and recombinant activated factor VII+platelets±antifibrinolytics in 67/829. The proportion of successful treatments to stop bleeding was 91.0% in cases treated with recombinant activated factor VII only, 82.7% for recombinant activated factor VII+antifibrinolytics, 72.7% for treatment with recombinant activated factor VII+platelets±antifibrinolytics, 78.8% for platelets±antifibrinolytics and 84.7% for antifibrinolytics alone. Treatment failure was documented in 18 bleeding events (2% of the total treatments), the majority of which were in patients receiving treatment with antifibrinolytics; bleeding re-started in 6% of bleeds after initial effective treatment. Thirty-five adverse events were reported, none of which was a thromboembolic event. Among treatments that included recombinant activated factor VII, only one patient reported three possibly drug-related non-serious adverse events (nausea, dyspnea and headache). To conclude, non-surgical bleeds were common and often severe in Glanzmann thrombasthenia; both platelets and recombinant activated factor VII appeared to be effective, and with good safety profiles, for the treatment of non-surgical bleeds. This trial was registered at clinicaltrials.gov identifier: NCT01476423.
Introduction
Glanzmann thrombasthenia (GT) is a rare autosomal recessive bleeding disorder with a normal platelet count and morphology, but abnormal platelet function [defective glycoprotein (GP) IIb-IIIa complex (integrin αIIbβ3)]1–5 due to a genetic defect in the ITGA2B or ITGB3 gene encoding GPIIb and GPIIIa, respectively.6–8 GT patients commonly present in childhood with mucocutaneous bleeds (easy bruising, purpura, epistaxis, gingival bleeds or menorrhagia),2,9 and often experience bleeds after dental extraction, surgery, delivery and trauma. Although the severity of bleeding may vary, GT is considered a severe bleeding disease as at least 75% of patients require blood and/or platelet transfusions at least once in their life.2,9,10 While bleeding in GT can be treated by local hemostatic agents or antifibrinolytics,11 when these are ineffective, platelet transfusion is required. Repeated platelet transfusion may, however, result in the development of antibodies [to human leukocyte antigen (HLA) or to GPIIb/IIIa] which may render future platelet transfusions ineffective.2,12–14
Following the successful treatment of epistaxis in a GT patient,15 several studies suggested that recombinant activated factor VII (rFVIIa; NovoSeven®) may be effective in the treatment of bleeding and the perioperative management of surgery in GT.16–18 Notably, an international survey in GT patients treated for bleeds and surgical/invasive procedures showed good effectiveness and tolerability for rFVIIa given as bolus injections; the findings also allowed for a preliminary suggestion of an optimal dosing regimen (≥3 doses of ≥80 μg/kg given at intervals of ≤2.5 h) for the treatment of moderate-to-severe bleeding episodes.19 Based largely on these findings, in 2004 rFVIIa was approved by the European Medicines Agency (EMA) for the treatment and prevention of bleeding episodes and in surgery or invasive procedures in GT patients with antibodies to GPIIb/IIIa and/or HLA, and a past or present history of platelet refractoriness to platelet transfusions.20 To date, for GT patients who are not refractory, platelets are used as the first-line treatment.
As part of the approval, the EMA required the collection of post-marketing pharmacovigilance data on rFVIIa in GT. The observational, international, multicenter, web-based Glanzmann’s Thrombasthenia Registry (GTR) was, therefore, launched to prospectively gather and evaluate information on the different treatment modalities and their outcomes in “real-world” clinical practice.21 Collection of such registry data is key to improving the understanding and management of rare diseases such as GT, for which randomized clinical trials are difficult to perform.
This paper reports new, clinically relevant effectiveness and safety data on rFVIIa, as well as the other therapeutic options available (antifibrinolytics and platelets), for the treatment of non-surgical bleeds in patients with GT, using data obtained from the largest observational study of GT patients, the GTR.
Methods
Patients
From May 10, 2007, to December 16, 2011, prospectively collected online data were entered into the GTR on the effectiveness and safety (including thromboembolic events) of systemic hemostatic treatments used in clinics in patients with GT, including rFVIIa, platelet transfusions (P), and antifibrinolytics (AF) alone or in combination with other agents. All patients were treated according to local practices in an open-label manner, and no drugs were supplied for this registry. The main features of the GTR include: (i) standardized data collection using a customized web-based tool; (ii) a protocol-based registry conducted in accordance with general data protection laws and any local country requirements for conducting observational studies; and (iii) centralized data management overseen by an external expert panel composed of four physicians and by the international medical director of Novo Nordisk (the study sponsor). Protocol definitions and outcomes specified by the study sponsor, and post hoc classifications of bleed severity employed by the external expert panel (in response to requests by the EMA), are reported in Online Supplementary Table S1.
Only patients with a diagnosis of congenital GT were included in the GTR (see Online Supplementary Table S1). Patients with acquired thrombasthenic states caused by autoimmune disorders or medications were excluded. Refractoriness and the presence of antibodies were coded initially and assessed periodically as deemed important by the investigator. As tests for antibodies may not have been available at all centers, antibodies may also have been present in some patients classified as having refractoriness only.
Data handling
As this was an observational study, treatment was based on local practice and there were no set treatment protocols. All serious adverse events were monitored and data validated by a serious adverse event query process. The GTR expert group set up strictly controlled data collection procedures, and any data entry queries (identified by a hematologist) were made via a stringent query process to the participating sites. Clarification of all critical parameters collected was obtained and discussed thoroughly by the expert group.
Ethics committee approval
This study was conducted in accordance with the Declaration of Helsinki and Guidelines for Good Pharmacoepidemiology Practices, and each participating center complied with local ethical and/or regulatory requirements. Signed informed consent was obtained from all patients (or parents/legal guardians for minors not yet able to give consent).
Statistical analysis
The effectiveness analysis was based on all treatment-allocated patients for whom the effectiveness endpoint was known. All patients and all treatment episodes were included in the safety analysis. All evaluations were summarized with numerical variables [mean, standard deviation (SD), median, maximum and minimum], while categorical variables were summarized as numbers and percentages. No formal statistical comparisons were performed.
Results
Recruitment into the Glanzmann Thrombasthenia Registry and effectiveness and safety datasets
Data were collected from 1,086 admissions (883 for bleeding and 204 for surgery; one admission was considered as both); 218 patients with GT were enrolled from 45 sites in 15 countries worldwide (Figure 1). All 1,086 admissions were included in the safety analysis. However, for the effectiveness evaluations, review of the data showed that four admissions were adverse events and not treatments for bleeds and were, therefore, excluded from the evaluations (Figure 1). In addition, it was found that on several occasions multiple admissions contained information on the same bleeding episode; in these instances, the admissions were collapsed and one designated as the index event. In total, 64 admissions for bleeds were considered to be linked events and were collapsed into 22 index events. Lastly, two admissions previously considered as bleeding episodes were in fact minor surgical procedures, and were transferred for analysis of surgical procedures (Figure 1). In total, the post hoc secondary effectiveness analysis was performed using data from 829 admissions for non-surgical bleeding, and 206 surgical admissions (Figure 1).
Figure 1.
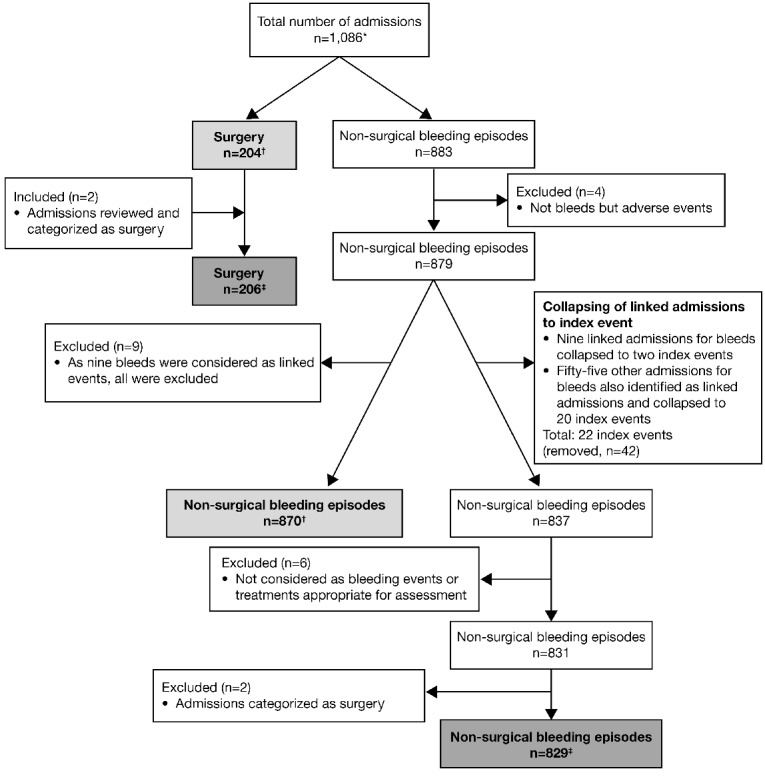
Chart depicting the flow of admissions into the GTR and the data used for primary and secondary analyses. *One admission was considered both a surgical procedure and a bleeding episode. †Data used for the primary effectiveness analysis (performed in 2012). ‡Data used for the secondary data analysis (performed in 2014). All data were included in the safety analysis.
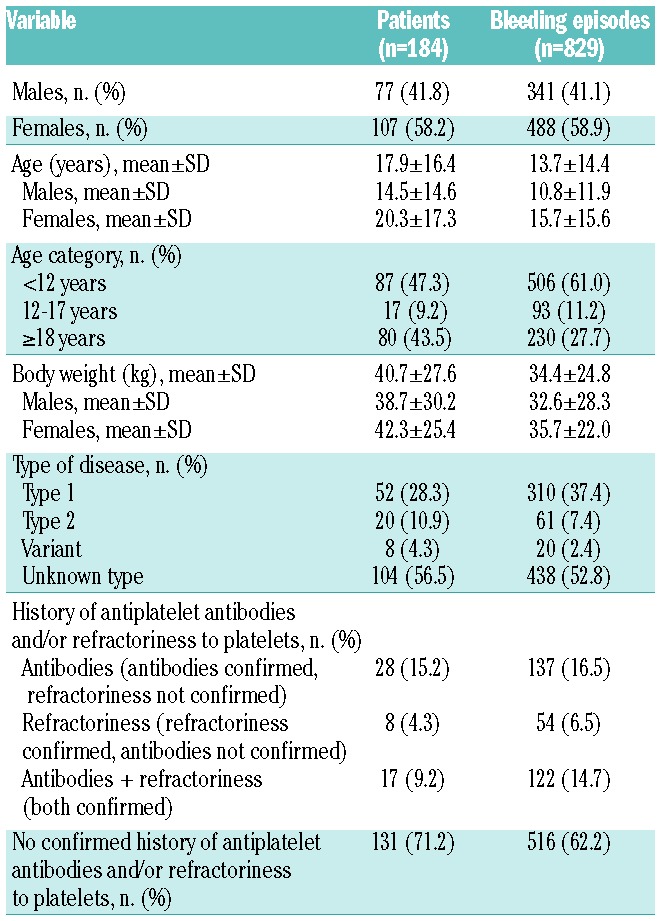
Of the 829 admissions for bleeding episodes, 216 (26.1%) were classified as severe and 613 (73.9%) as moderate bleeds, while 630 (76.0%) were spontaneous and 199 (24.0%) were post-traumatic bleeds (see Online Supplementary Table S2 for a detailed description of the bleeding episodes reported). Clinical and demographic characteristics of the patients who experienced non-surgical bleeds are presented in Table 1.
Table 1.
Glanzmann Thrombasthenia Registry: clinical and demographic characteristics of the population at admission.
Dosing/scheduling
Regardless of the severity of the bleeding, P±AF was the most commonly used treatment (Online Supplementary Figure S1). Of the 829 bleeding episodes, 312 (37.6%) were treated with P±AF, 219 (26.4%) with AF only, 124 (15.0%) with rFVIIa alone, 107 (12.9%) with rFVIIa+AF and 67 (8.1%) with rFVIIa+P±AF. Concomitant AF treatment was documented in 46.3% of bleeds treated with rFVIIa, in 67.9% of those treated with P and in 79.1% of bleeds treated with rFVIIa+P.
Investigators reported that for the 313/829 bleeds that occurred in patients with a history of antiplatelet antibodies and platelet refractoriness, antibodies only or refractoriness only, P±AF was used in 45.4% (51 severe and 91 moderate), rFVIIa+AF in 19.2% (32 severe and 28 moderate bleeds), AF alone in 14.4% (8 severe and 37 moderate), rFVIIa+P±AF in 11.5% (23 severe and 13 moderate) and rFVIIa alone in 9.6% of cases (all moderate bleeds). The use of rFVIIa alone, or together with AF (20.4% and 31.5%, respectively) to treat bleeding episodes, was most frequent in patients with a history of refractoriness alone, while AF was mainly used to treat bleeds that occurred in patients with no history of antiplatelet antibodies and/or platelet refractoriness (Online Supplementary Figure S2).
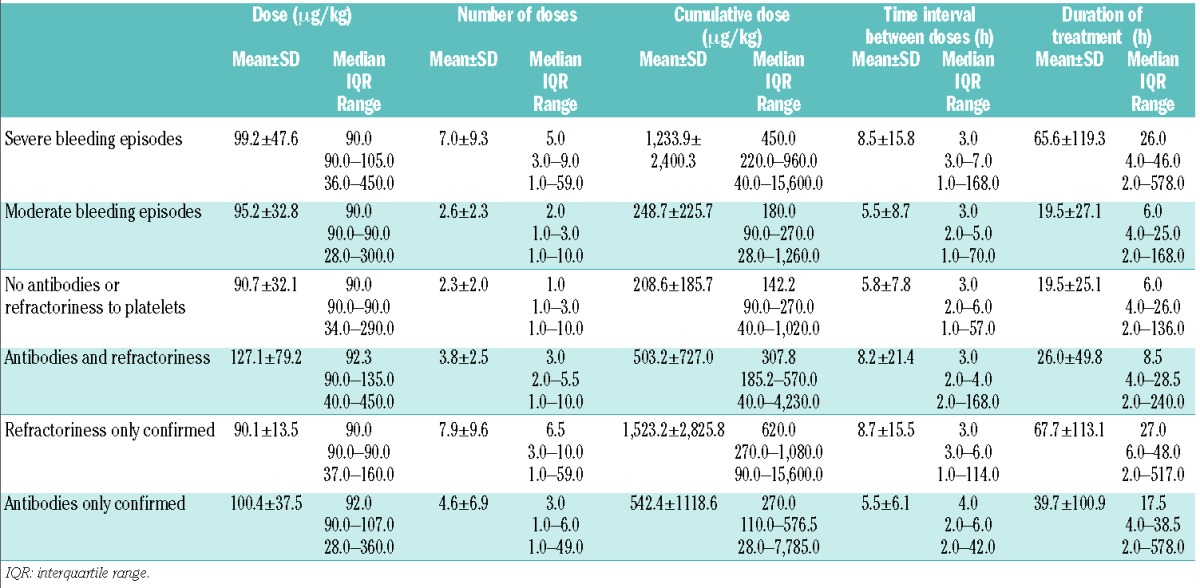
The number of rFVIIa doses, cumulative dose and overall duration of treatment were greater in patients with severe bleeds than in those with moderate bleeds, and in patients with a history of antiplatelet antibodies and/or refractoriness to platelets than in subjects without such a history (Table 2).
Table 2.
Glanzmann Thrombasthenia Registry: doses and duration of treatment with rFVIIa in the GTR stratified according to bleeding severity and to a history of refractoriness/antibodies (n=298).
Evaluation of treatment effectiveness
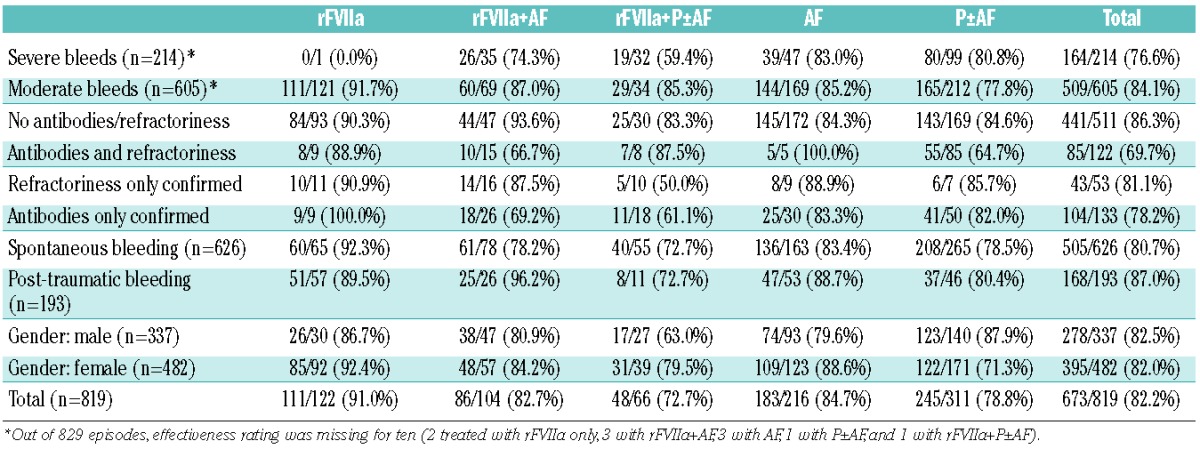
Overall, treatment was judged to be effective (see Online Supplementary Table S1 for definition) in 91.0% of bleeds treated with rFVIIa alone, 84.7% of those treated with AF alone, 82.7% of those treated with rFVIIa+AF, 78.8% of those treated with P±AF and 72.7% of those treated with rFVIIa+P±AF (Table 3). For 164 bleeds treated with rFVIIa (either alone, with AF or with platelets) for which information on treatment duration was available, the total duration of the treatment with rFVIIa was <6 h for 76 (46.3%) bleeding episodes. Out of these 76 bleeding episodes, 15 (19.7%) were severe and 34 (44.7%) occurred in patients with a history of antibodies and/or refractoriness; the treatment of 66 of the 76 bleeding episodes (86.8%) was rated “effective.”
Table 3.
Glanzmann Thrombasthenia Registry: rate of full effectiveness of different treatment options stratified according to demographic and clinical characteristics of the bleeding episode and to a history of antiplatelet antibodies and/or refractoriness to platelets (n=819).
Treatment failure
In 18 cases (2.2% of the total: 5 severe, 13 moderate bleeds: 8 in patients without and 10 in patients with a history of antibodies/refractoriness), treatment failure was documented. With respect to the initial treatment employed, 9/18 cases were receiving AF, 5/18 cases were receiving P±AF, 3/18 were receiving rFVIIa+AF and one case was receiving rFVIIa+P±AF.
Re-bleeding
For 738 episodes for which data on the re-bleeding status were available, 45 re-bleeds in 25 patients were registered: 19/189 (10.1%) severe bleeding episodes, 26/549 (4.7%) moderate bleeding episodes, 18/478 (3.8%) bleeds in patients without antibodies or refractoriness and 27/260 (10.4%) bleeding episodes in patients with a history of antiplatelet antibodies and/or refractoriness to platelets. Re-bleeding occurred in 23/284 (8.1%) bleeds treated with P±AF, in 4/202 (2%) of those treated with AF alone, in 12/57 (21.1%) of those treated with rFVIIa+P±AF, in 1/105 (0.95%) of those treated with rFVIIa alone and in 5/90 (5.6%) of bleeding episodes treated with rFVIIa+AF.
Safety data
Forty-six adverse events in 18 patients were reported in the registry, of which 15 were serious adverse events. In a total of 15 adverse events (in 9 patients), the treatment included rFVIIa. Seven serious adverse events in the GTR were entries for adverse events only, all unrelated to rFVIIa treatment, and were as follows: six adverse events in one patient (angina, alteration of clinical status, hyperthermia, facial and eyelid edema, and staphylococcus infection), and one serious adverse event in a second patient (viral meningitis). Table 4 summarizes the adverse events reported in treated patients.
Table 4.
Summary of adverse events reported in treated patients in the GTR*.
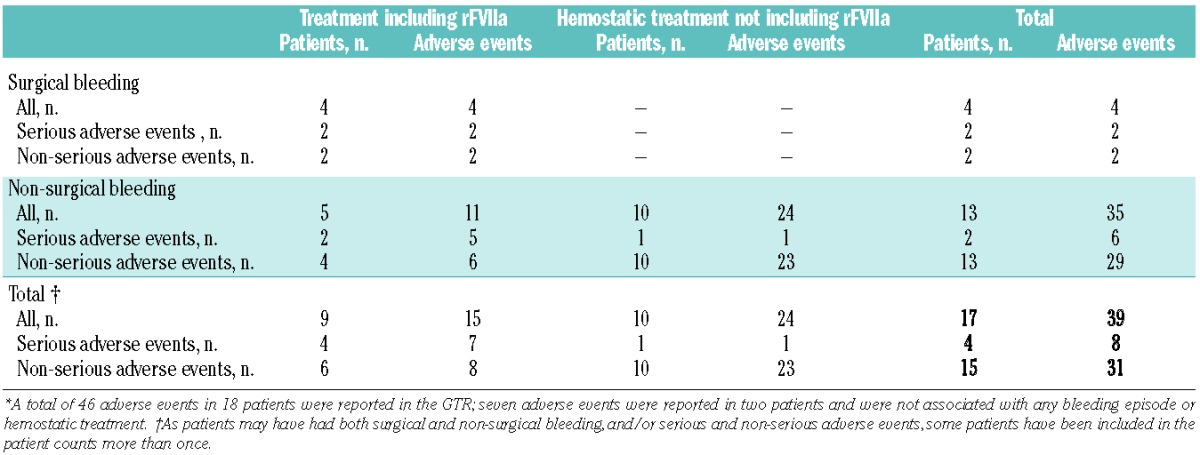
For the non-surgical bleeding results presented in this article, 35 adverse events were reported (including 6 serious adverse events; Table 4). For bleeds in which patients received rFVIIa, 11 adverse events occurred, five of which were serious adverse events (subarachnoid bleeding, septicemia, respiratory insufficiency, cardiac decompensation, and re-bleeding and hematoma due to a fall) and six were non-serious (bacterial infection, fever, two headaches, nausea and dyspnea). Eight of the 11 adverse events were judged by the investigators to be unlikely to be related to rFVIIa treatment, while three adverse events in one patient (nausea, dyspnea and headache) were judged to be probably or possibly related to rFVIIa. No thromboembolic events were reported for any of the treatments for non-surgical bleeds and no unexpected laboratory values were reported.
Discussion
The GTR data presented here come from the largest observational study reporting on GT patients and address issues that remained following a previous survey of GT patients19 in which the use of hemostatic agents other than rFVIIa was not included. The findings show that GT patients can present with severe bleeds and approximately one-third of the bleeds reported here occurred in patients with a history of platelet antibodies and/or refractoriness. Recombinant FVIIa, and other currently available treatments for bleeding in GT, were found to have good safety and effectiveness profiles in most patients.
One potential concern regarding the use of rFVIIa in GT patients is whether treatment may be thrombogenic. The GTR data show that when administered alone or in combination with platelets or antifibrinolytics, rFVIIa treatment of non-surgical bleeding episodes was not associated with any thromboembolic events in GT patients. Together with its long shelf-life and ready availability, rFVIIa appears to be an attractive treatment option to administer early (as first-line treatment) for non-surgical bleeds in all GT patients while waiting for HLA-compatible platelet concentrates or for concentrates prepared from single-donor apheresis.
Regardless of the severity of bleeding or history of antiplatelet antibodies and/or refractoriness to platelets, the median rFVIIa dose and dosing interval aligned with those recommended previously for GT patients (≥80 μg/kg, ≤2.5 h interval).19 In all groups of patients, the number of doses reported for severe bleeds was understandably higher than for moderate bleeds, and severe bleeds were also associated with longer treatment duration and higher cumulative doses.
Currently, platelets are the first-line therapy for severe bleeding in GT patients; as previously reported,22 platelets were also used in the GTR in patients with a history of platelet antibodies and/or refractoriness. The effectiveness of platelets in these patients may be due to some patients no longer being refractory or to inhibitory antibodies no longer being present. While few data on the natural history of inhibitors in GT are available, it has been suggested that the effectiveness of platelets in such a setting may be related to the long time intervals between exposure to platelets in GT, and perhaps also to the transient nature of the inhibitors.
Bleeds in GT patients may be treated with antifibrinolytics first (possibly in the home setting); then, if these are not effective enough, other treatments may be introduced (rFVIIa and/or platelets) during the treatment of the bleeding episode. Data from the GTR suggesting that treatment with antifibrinolytics alone was effective may be a reflection of this clinical practice. In general, the prospective nature of the registry and the less-controlled data collection process compared with actual clinical trials make it inherently difficult to interpret or directly compare effectiveness results between treatments.
Limitations
As consecutive individuals with the same inclusion/exclusion criteria were enrolled in the GTR, in addition to the observational, prospective nature of the registry and the less-controlled data collection process compared with clinical trials, it is inherently difficult to interpret or directly compare effectiveness results between treatments. A further limitation of the present study may pertain to the arbitrary classification of severe and moderate bleeds. However, such simple definitions were employed at the request of the EMA and are similar to the definitions of “major and minor bleeds” published in April 2005 by the Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis.23 Lastly, the coding of a history of refractoriness or antibodies was performed at the time of the first admission and when the investigator deemed appropriate. This lack of documentation of specific anti-body testing or refractoriness at the time of a particular episode limits the analysis (particularly for use of platelet-based regimens).
Acknowledgments
Editorial assistance was provided by Sharon Eastwood (PAREXEL) and the study was financially supported by Novo Nordisk Healthcare AG. The authors would also like to thank Soraya Benchikh el Fegoun and Jens Bjerre (Novo Nordisk Healthcare AG) for their comments and advice, Anders Rosholm (Novo Nordisk Healthcare AG) for statistical support and Yves Laurian who acted as an expert panel member for the GTR until 2008.
Footnotes
The online version of this article has a Supplementary Appendix.
GTR participating investigators.
Algeria: Grifi Fathia, Sidi Mansour Nourredine, Mesli Naïma, Hamdi Selma, Belhani Meriem, Touhami Hadj; Austria: Max Heistinger, Paul Alexander Kyrle; Belgium: Christel Van Geet, Veerle Labarque; Bulgaria: Angelina Stoyanova, Katya Sapunarova; France: Caroline Oudot, Roseline d’Oiron, Gruel Yves, Albert Faradji, Achille Aouba, Nathalie Trillot, Alain Marquès-Verdier, Claire Pouplard; Germany: Rainer B Zotz, Roswith Eisert (replaced by Ingvild Birschmann), Mario von Depka Prondzinski, Maximillian Kirchmaier, Markus Rieke, Daniele Pillitteri; Hungary: Agota Schlammadinger, Csongor Kiss, Laszlo Nemes; Italy: Gavino Piseddu, Giovanni Di Minno, Antonio Coppola, Paola Giordano, Elisabetta Sacchi, Michele Schiavulli; The Netherlands: Paula Frouke Ypma, Meijer Karina, Maria Kruip, Pieter Kamphuisen, Britta Laros Van Gorkom, Karly Hamulyak, Rienk Yde Johan Tamminga; Pakistan: Tahir Shamsi, Munira Borhany; Spain: Rosario Perez Garrido, Victor Jiménez-Yuste; Sweden: Erik Berntorp, Karin Knobe; Switzerland: Dimitrios Tsakiris, Brigitte Brand; UK: John D Grainger, Kate Khair, Jayashree Motwani, Paula Bolton-Maggs; USA: Marcella Torres.
Funding
Novo Nordisk Health Care AG financially supported the GTR in compliance with international guidelines for Good Publication Practice. Novo Nordisk Health Care AG also supported several authorship meetings held to plan and discuss the manuscript and its content.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Caen JP, Castaldi PA, Leclerc JC, et al. Congenital bleeding disorders with long bleeding time and normal platelet count. 1. Glanzmann’s thrombasthenia (report of fifteen patients). Am J Med. 1966;41(1):4–26. [Google Scholar]
- 2.George JN, Caen JP, Nurden AT. Glanzmann’s thrombasthenia: the spectrum of clinical disease. Blood. 1990;75(7):1383–1395. [PubMed] [Google Scholar]
- 3.Nurden AT, Caen JP. An abnormal platelet glycoprotein pattern in three cases of Glanzmann’s thrombasthenia. Br J Haematol. 1974;28(2):253–260. [DOI] [PubMed] [Google Scholar]
- 4.Nurden AT. Inherited abnormalities of platelets. Thromb Haemost. 1999;82(2):468–480. [PubMed] [Google Scholar]
- 5.Reichert N, Seligsohn U, Ramot B. Clinical and genetic aspects of Glanzmann’s thrombasthenia in Israel: report of 22 cases. Thromb Diath Haemorrh. 1975;34(3):806–820. [PubMed] [Google Scholar]
- 6.Kannan M, Ahmad F, Yadav BK, Kumar R, Choudhry VP, Saxena R. Molecular defects in ITGA2B and ITGB3 genes in patients with Glanzmann thrombasthenia. J Thromb Haemost. 2009;7(11):1878–1885. [DOI] [PubMed] [Google Scholar]
- 7.Nurden AT, Nurden P. Inherited disorders of platelets: an update. Curr Opin Hematol. 2006;13(3):157–162. [DOI] [PubMed] [Google Scholar]
- 8.Nurden AT, Fiore M, Nurden P, Pillois X. Glanzmann thrombasthenia: a review of ITGA2B and ITGB3 defects with emphasis on variants, phenotypic variability, and mouse models. Blood. 2011;118(23):5996–6005. [DOI] [PubMed] [Google Scholar]
- 9.Toogeh G, Sharifian R, Lak M, Safaee R, Artoni A, Peyvandi F. Presentation and pattern of symptoms in 382 patients with Glanzmann thrombasthenia in Iran. Am J Hematol. 2004;77(2):198–199. [DOI] [PubMed] [Google Scholar]
- 10.Franchini M, Favaloro EJ, Lippi G. Glanzmann thrombasthenia: an update. Clin Chim Acta. 2010;411(1–2):1–6. [DOI] [PubMed] [Google Scholar]
- 11.Di Minno G, Coppola A, Di Minno MN, Poon MC. Glanzmann’s thrombasthenia (defective platelet integrin alphaIIb-beta3): proposals for management between evidence and open issues. Thromb Haemost. 2009;102(6):1157–1164. [DOI] [PubMed] [Google Scholar]
- 12.Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol. 2008;142(3): 348–360. [DOI] [PubMed] [Google Scholar]
- 13.Martin I, Kriaa F, Proulle V, et al. Protein A Sepharose immunoadsorption can restore the efficacy of platelet concentrates in patients with Glanzmann’s thrombasthenia and anti-glycoprotein IIb-IIIa antibodies. Br J Haematol. 2002;119(4):991–997. [DOI] [PubMed] [Google Scholar]
- 14.Rebulla P. A mini-review on platelet refractoriness. Haematologica. 2005;90(2):247–253. [PubMed] [Google Scholar]
- 15.Tengborn L, Petruson B. A patient with Glanzmann thrombasthenia and epistaxis successfully treated with recombinant factor VIIa. Thromb Haemost. 1996;75(6):981–982. [PubMed] [Google Scholar]
- 16.Almeida AM, Khair K, Hann I, Liesner R. The use of recombinant factor VIIa in children with inherited platelet function disorders. Br J Haematol. 2003;121(3):477–481. [DOI] [PubMed] [Google Scholar]
- 17.D’Oiron R, Menart C, Trzeciak MC, et al. Use of recombinant factor VIIa in 3 patients with inherited type I Glanzmann’s thrombasthenia undergoing invasive procedures. Thromb Haemost. 2000;83(5):644–647. [PubMed] [Google Scholar]
- 18.Poon MC, Demers C, Jobin F, Wu JW. Recombinant factor VIIa is effective for bleeding and surgery in patients with Glanzmann thrombasthenia. Blood. 1999;94(11):3951–3953. [PubMed] [Google Scholar]
- 19.Poon MC, D’Oiron R, Von Depka M, et al. Prophylactic and therapeutic recombinant factor VIIa administration to patients with Glanzmann’s thrombasthenia: results of an international survey. J Thromb Haemost. 2004;2(7):1096–1103. [DOI] [PubMed] [Google Scholar]
- 20.NovoSeven® summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000074/WC500030873.pdf Accessed July 23, 2014.
- 21.Poon MC, Zotz R, Di Minno G, Abrams ZS, Knudsen JB, Laurian Y. Glanzmann’s thrombasthenia treatment: a prospective observational registry on the use of recombinant human activated factor VII and other hemostatic agents. Semin Hematol. 2006;43(1 Suppl 1):S33–S36. [DOI] [PubMed] [Google Scholar]
- 22.Santoro C, Rago A, Biondo F, et al. Prevalence of allo-immunization anti-HLA and anti-integrin alphaIIbbeta3 in Glanzmann thromboasthenia patients. Haemophilia. 2010;16(5):805–812. [DOI] [PubMed] [Google Scholar]
- 23.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–694. [DOI] [PubMed] [Google Scholar]


