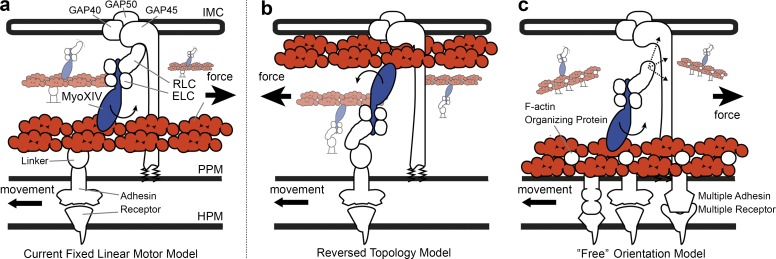
Figure 2.
Alternative models for the apicomplexan gliding motor organization. Schematics for three alternative gliding motor models. (a) The fixed linear motor model, based on Soldati et al. (2004), the most widely accepted current model responsible for the mechanics of gliding and invasion. (b) An alternative reversed topology model from King (1988) developed before isolation of the GAP protein complex and now less widely considered. (c) A “free” motor model that the authors support, in which actin filaments are bound to the underlying PPM directly (avoiding a linking protein) and myosin motor orientation is not fixed along the anterior–posterior axis. Proteins labeled include the glideosome-associated proteins, GAP40, 45, and 50. GAP45 spans the IMC space embedded in both membranes via palmitoylation and myristoylation. In each model, myosin XIV is shown with its neck bound by essential and regulatory light chains (ELC and RLC, the latter also called MTIP in Plasmodium or MLC in Toxoplasma). F-actin is shown as a red doublet helical polymer. In model a, and with aldolase no longer believed to play a mechanical role (Shen and Sibley, 2014), an unknown linker is shown connecting actin filaments to the cytoplasmic domain of a surface-bound adhesin (e.g., a TRAP-like protein). Model b requires a reversed polarity of actin filaments so that myosin-generated motor force correlates with forward motion. In our alternative model, c, actin is arranged into filament bundles by organizing proteins, such as Coronin (Olshina et al., 2015; Bane et al., 2016). Because the direct interaction between the myosin XIV motor complex and GAP45/GAP-protein complex is unknown, this is presented with dashed arrows and question marks. This means the direction of motor force is not fixed to the anterior–posterior axis. Instead directional movement would rely on other factors such as cell shape (restricting direction of movement) or be entirely reliant on F-actin orientation (restricted to those myosin heads oriented to produce viable motor force). An additional innovation of this model is that motor force generated by myosin XIV moves a patch of membrane (F-actin bound) in which multiple adhesins are embedded. Links to the extracellular substrate are left intentionally ambiguous in light of conflicting evidence for the role played by previously implicated candidates in transmitting force (Bargieri et al., 2013; Riglar et al., 2016). HPM, host plasma membrane.