The Saudi Association for the Study of Liver Diseases and Transplantation (SASLT) formed a task force to evaluate the current methods of optimal management of the Hepatitis C virus (HCV) infection in Saudi Arabia. All members of this committee are hepatologists.
The first step was to undertake a broad literature search of the published literature on every aspect of HCV management. All available literature on the topic was critically examined, and the available evidence was then classified according to its importance.
The contents of the resulting document, including the recommendations contained in it, have been discussed in detail and agreed upon by the members of the SASLT task force. Subsequently, and after review by the board of directors, the guidelines were approved and endorsed by SASLT.
All recommendations in these guidelines are based on the best available evidence, and tailored to patients treated in Saudi Arabia. They are graded on the basis of evidence.
The purpose of these guidelines is to improve HCV patient care in the Kingdom and to promote and improve the multidisciplinary care required in the treatment of these patients. They are intended for use by physicians and offer the recommended approaches to treatment of HCV with the new direct-acting antiviral treatment.
Grading of recommendations based on quality of evidence
Grade A: Recommendation based on at least one high quality randomized controlled trial or at least one high quality meta-analysis of methodologically sound randomized controlled trials.
Grade B: Recommendation based on high quality case-control or cohort studies or a high quality systematic review.
Grade C: Recommendation based on nonanalytic studies (case reports or case series).
Grade D: Recommendation based on expert opinion only.
The strength of each recommendation can be divided into:
Level 1: strong, based on quality of evidence, patient outcome, and cost
Level 2: weak, with variability in values, preferences, and less certainty.
Goals of these guidelines
These are as follows:
To complement the previous SASLT guidelines in the management of hepatitis C in Saudi Arabia
To provide an evidence-based approach for the management of HCV-infected patients
To eradicate HCV infection. Succeeding in this aim would result in a decrease in liver-related complications, deaths, the need for liver transplantations, and hepatocellular carcinoma rate.
INTRODUCTION
Hepatitis C virus (HCV) has been reported to be on the decline over the past decade, although it remains a major public health concern in Saudi Arabia. Its prevalence in Saudi Arabia is generally uncertain because most studies were conducted more than 10 years ago. However, data from blood donor screening centers indicates prevalence rates of 0.4–1.1%.[1] The premarital screening data in a predominantly young population from a survey among 74662 individuals conducted in the period between January and May 2008, the results of which were published by the Ministry of Health, showed an HCV prevalence of only 0.33%.[2] Similarly, a community-based study in 16–18 years old Saudi adolescents in 2008 showed a prevalence of HCV at 0.22% in the group.[1]
The most prevalent genotype is genotype (GT) 4, followed by GT1. HCV GT4 accounts for 60% of the cases, GT1 for 25.9%, GT2 for 4.3%, GT3 for 2.9%, and GT5/GT6 for 0.3%. 6.3% of the cases were of mixed genotypes, predominantly between GT1 and GT4.[3] The most common subtypes of GT4 are 4a (48%) and 4d (39%), followed by subtypes 4n (6%) and others (6%).[4] Up to 63% of Saudi patients have minimal to moderate (Metavir, F0–2) histological disease.[5]
DIAGNOSIS OF HCV
Detection of the anti-HCV antibody is the method used for screening of HCV infection. Enzyme immunoassays (EIAs) is the commonly used test, with a specificity of >99% in the detection of anti-HCV.[6] EIA can detect HCV antibodies as early as 6–8 weeks after exposure.[7] Overall, HCV antibody tests have a strong positive predictive value for exposure to the HCV. If anti-HCV antibodies are detected, HCV RNA should then be determined by a sensitive molecular method such as polymerase chain reaction (PCR), transcription mediated amplification (TMA), or branched DNA (b-DNA) with a lower limit of detection of <15 international units (IU)/ml. All HCV nucleic acid molecular tests have the capacity to detect the presence of the virus and to measure the amount of the virus present in the blood (the HCV viral load). Viral RNA testing is also indicated when there is a clinical suspicion of HCV, transaminase levels are high, and antibody testing is negative.[8] HCV genotype and subtype can be determined via various methods, including direct sequence analysis, reverse hybridization, and genotype-specific real-time PCR.[9] Genotyping is useful in epidemiological studies, in selecting therapy, predicting the likelihood of response to the chosen therapy and determining the optimal duration of treatment.
Noninvasive laboratory tests to assess liver fibrosis
Various noninvasive tests are being investigated for staging the degree of liver fibrosis. These tests may be used to decide whether to initiate or to delay the antiviral therapy and to monitor the effects of such therapy.[10]
The use of biochemical markers of liver fibrosis (Fibrotest) and necrosis (ActiTest) can be recommended as an alternative to elastograms and liver biopsy for the assessment of liver injury in patients with chronic hepatitis C. Both have been shown to accurately identify patients with mild fibrosis or cirrhosis. However, they have also been shown to be less effective in discriminating between moderate and severe fibrosis.[11]
Transient elastography (Fibroscan)
Fibroscan is a technique used to assess liver stiffness without any invasive procedure. The scan can be performed easily, produces no side effects, and is an inexpensive procedure. Fibrosis in the liver can be quantified using elastography. Transient elastography is performed using transducer-induced vibrations at low frequency and amplitudes. Tissue elasticity is detected through pulse-echo ultrasound, which measures shear wave velocity, the S-wave. The wave travels faster in less elastic and stiff livers such as those found in patients with advanced liver fibrosis. Results of liver elasticity are expressed in kilopascals (kPa).
A liver stiffness measurement using Fibroscan is reproducible and independent of the operator and explores a volume of liver parenchyma, which can be approximated to a cylinder of 1 cm in diameter and 4 cm in length. This volume is 100 times larger than the biopsy specimen size, and is thus much more representative of the entire hepatic parenchyma.[12] Some extensive studies have demonstrated that the measurement of liver stiffness with Fibroscan is a real alternative to liver biopsy. The amount of fibrosis can be quantified very easily and reliably, and is feasible in more than 95% of the patients. However, the accuracy of the test is hampered by obesity, ascites, and narrow intercostal spaces. Acute hepatitis and liver congestion such as that found in cardiac failure can cause false high scores. Sometimes it may be virtually impossible to take measurements in such patients.[12] Fibroscan values range from 2.4 to 75.5 kPa with cutoff values of 7.1 kPa for F ≥ 2, 9.5 kPa for F ≥ 3 and 12.5 kPa for F = 4 (according to the Metavir histological classification system).[12,13] In a study comparing elastography to histological examination on 327 patients, it was concluded that liver stiffness measurements and fibrosis grades were well correlated, with increasing reliability in more extensive fibrosis (F ≥ 3) or cirrhosis. It was impossible to determine a cutoff value to differentiate between F0 and F1 by Fibroscan.[12,14]
Histology
Liver biopsy still remains the gold standard test for evaluating the stages of fibrosis, and, when combined with clinical and laboratory findings, it is also a reliable means of assessing prognosis, thus helping to provide information about the need to initiate therapy. However, biopsy is not mandatory to initiate therapy.[15]
Recommendations
Diagnosis of HCV infection is based on the detection of anti-HCV antibodies by enzyme immunoassay and HCV RNA by a sensitive molecular method (lower limit of detection ≤ 15 IU/ml), ideally a real-time PCR assay (grade A1)
In immunosuppressed patients with undetectable anti-HCV antibodies and in cases of suspected acute hepatitis, HCV RNA test should be a part of initial evaluation (grade A1)
Determination of HCV genotype and subtype is recommended and should be used to determine the choice of therapy and duration of treatment (grade A1)
Transient elastography can be used to assess liver fibrosis in patients with chronic hepatitis C provided that consideration is given to factors that may adversely affect its performance, such as obesity, age, and biochemical necroinflammatory activity (grade A1)
The use of biochemical markers of liver fibrosis (Fibrotest) and necrosis (ActiTest) can be recommended as an alternative to transient elastography and liver biopsy for the assessment of liver injury in patients with chronic hepatitis C (grade A1)
Liver biopsy is valuable for assessing the status and level of liver inflammation, the potential progression of fibrosis, and the presence or absence of cirrhosis. It is not mandatory, however, and should be reserved for conditions where there is uncertainty or additional diseases need to be ruled out (grade A1).
TREATMENT OF HEPATITIS C VIRUS INFECTION
The development of direct-acting antiviral drugs
HCV is a small RNA virus consisting of a viral genome—a positive sense, single-stranded RNA—enclosed in a nucleocapsid, or capsid shell, and surrounded by viral envelope E1 and E2, a lipid membrane in which glycoproteins are anchored [Figure 1].[16,17] Since the discovery of HCV in 1989,[18] a tremendous amount of research has been undertaken and recorded, which has helped to improve our understanding of HCV virology. Some of the major tools used in this research have included replicon systems—synthetic genetic constructs in which some or all of the HCV genes are allowed to replicate in cell cultures[19]—which have improved the understanding of HCV genomic replication, and retrovirus-based pseudotyped particles,[20] which in turn have improved the understanding of virus entry. The development of a replicon model was a particular turning point in HCV research, considerably expanding the possibilities for studying viral replication and screening potential anti-HCV drugs for activity against viral enzymes. Since 2005, the full HCV lifecycle has also been investigated with the help of complete viral replication systems.[21,22] The HCV life cycle involves several steps: (1) host cell attachment, entry, and uncoating; (2) translation of the HCV genome into viral proteins; (3) cleavage and processing of viral proteins; (4) replication of HCV genome; (5) and assembly of new virions and release from host cell.
Figure 1.
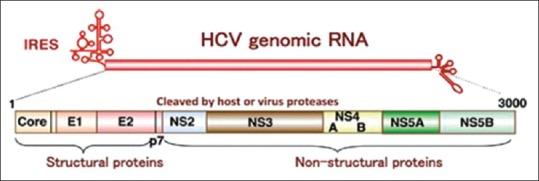
HCV genome organization. The HCV Open Reading Frame encodes three structural proteins, a small protein p7 ion channel, and 6 non-structural (NS) proteins. The structural proteins consist of core (c) proteins and envelop E proteins. The nonstructural proteins consist of NS2, NS3, NS4A, NS4B, NS5A, and NS5B types. Together, NS3/4A, NS4B, NS5A, and NS5B constitute the viral proteins of the replication machinery, which replicates the positive sense RNA genome through a negative strand intermediate. The viral RNA-dependent RNA polymerase NS5B is the key enzyme of RNA synthesis
The treatment of HCV has also progressed over the last 25 years since its discovery. In 1991, the first alfa interferon (IFN-α) was approved for the treatment of hepatitis C. The rates of sustained virologic response (SVR24) were extremely poor, however, and reported to be only 9% for GT1 and 30% for GT2 and GT3. Treatment responses were improved from 1998, with the addition of ribavirin (RBV)[23] (29% SVR for GT1 and 62% for GT2 and 3) and then improved again (to 41–51% SVR for GT1 and 70-82% for GT2 and GT3) in 2001, by linking the IFN (IFN) molecule to polyethylene glycol[24] (PegIFN). Recently, there has been another major breakthrough in hepatitis C treatment with the licensing of the first Direct-Acting Antiviral (DAAs) [Table 1]. These drugs directly target HCV's nonstructural replication machinery proteins (NS3/4A, NS5A, and NS5B), leading to the disruption of HCV replication. The first-generation and first-wave protease inhibitors (PIs) telaprevir and boceprevir were indicated only for GT1 HCV infection, requiring that they be administered in combination with PegIFN-α and RBV as a triple regimen, with estimated SVR results between 65% and 75%.[25,26] However, significant drug-adverse events, the complexity of the treatment response-guided regimen, the necessity of PegIFN, the narrow spectrum, and the low genetic barrier of resistance were all major disadvantages associated with the use of these drugs. Moreover, the reported SVR results were far inferior to those of the second-wave DAAs, particularly in difficult to treat populations such as cirrhotics, human immunodeficiency virus (HIV), and organ transplant patients. Consequently, neither drugs are currently indicated for the treatment of HCV infection.
Table 1.
Currently approved direct-acting drugs
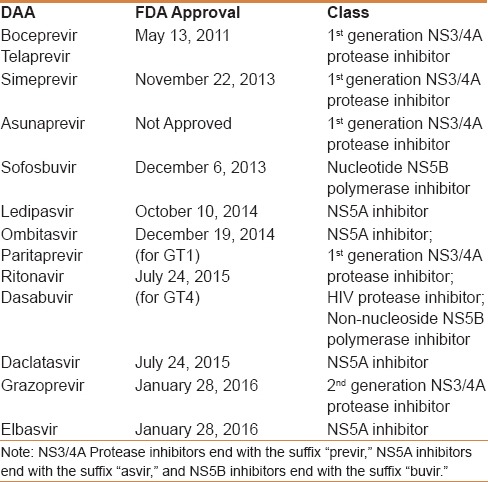
The approval of second-wave DAAs in November and December 2013 set new standards of care for HCV patients. By October 2014, the first INF-free “all-oral regimens” became available, substantially increasing the SVR results to more than 90%. These second-wave DAAs are characteristically associated with favorable drug-safety profiles, shorter treatment durations, superior SVR results, the availability of an INF-free option, and an ability with some regimens to treat HCV in a wide spectrum of conditions, including decompensating cirrhotics, liver transplants, renal, and HIV patients, with excellent results. The currently available DAAs are classified based on the site of the mechanism of action as:
NS3-4A PIs that bind to the catalytic site of the enzyme and block post-translational processing of viral polyproteins, preventing the release of functional, nonstructural proteins. First-generation PIs include telaprevir, boceprevir, simeprevir (SMV), ritonavir-boosted paritaprevir (PTV), and asunaprevir, and a second-generation PI is grazoprevir (GZR)
NS5A inhibitors that bind to domain 1 of the NS5A protein dimer and block its ability to regulate HCV replication within the replication complex. They also inhibit the assembly and release of viral particles. First-generation NS5A inhibitors include daclatasvir (DCV), ledipasvir (LDV), ombitasvir (OBV), and elbasvir (EBR)
Non-nucleoside NS5B polymerase inhibitors that bind to one of four allosteric sites of the RNA-dependent RNA polymerase (RdRp). By altering the conformation of the RdRp, they block its catalytic function, thereby indirectly blocking RNA replication. An example of a non-nucleoside NS5B polymerase inhibitor is dasabuvir (DSV)
Nucleotide NS5B polymerase inhibitors that act as false substrates for HCV RdRp, resulting in chain termination after being incorporated into the newly synthesized viral RNA. An example of a nucleotide NS5B polymerase inhibitor is sofosbuvir (SOF).
The objectives of hepatitis C virus treatment
The primary objective of HCV treatment is to cure hepatitis C infection. An SVR[27] is defined as being when HCV RNA is undetectable 12 weeks (SVR12) after treatment completion, thus indicating cure from infection in more than 99% of patients.[28] The hepatic benefits[29] of getting SVR are considerable, and include histologic regression of necroinflammation and liver fibrosis,[30] as well as reduced risk of complications, such as hepatic failure and portal hypertension. Moreover, the risk of hepatic cell carcinoma (HCC) in cirrhotic patients is reduced, though not eliminated, and all-cause mortality is significantly reduced.[31,32]
Recommendation
-
7.
The primary objective of treating HCV infected individuals is virological cure as defined by SVR. Elimination of HCV is associated with reduced all-cause mortality and liver related complications (grade A1).
Indications and contraindications for hepatitis C virus therapy with direct acting antivirals
Indications for therapy
DAA treatments of HCV are indicated in all adult patients with active HCV infection, and priority should be given to the following types:
Patients with advanced fibrosis (F3) or cirrhosis (F4) including decompensated cirrhosis
Patients with HIV or hepatitis B virus (HBV) coinfection
All solid organ transplant recipients with HCV RNA positive including patients with recurrence after liver transplantation
Patients with extrahepatic HCV-related complications such cryoglobulinemia vasculitis, HCV-related renal disease, or HCV-related malignancy
Females of childbearing age who wish to get pregnant
Patients discovered to have active HCV at a premarital screening program, irrespective of their disease stage.
Contraindications
DAA treatments of HCV are contraindicated in:
Patients who are less than 18 years old
Pregnant or lactating patients or couples unwilling to comply with adequate contraceptive measures
HCV patients with a life expectancy ≤1 years
Patients with hypersensitivity to any component of the formulation
Potential major drug–drug interaction between the DAA HCV medication and another vital medication that cannot be changed or stopped by the patient for any reason
Patients with Child Pugh B/C cirrhosis should not receive SMV, PTV/OBV and/or DSV or EBR/GZR as HCV therapy
Patients with severe renal impairment (CrCl < 30 mL/min) or patients on hemodialysis should avoid sofosbuvir-based therapy.
DRUG–DRUG INTERACTION WITH DIRECT-ACTING ANTIVIRALS
With the revolution in HCV treatment and the development of strong and efficacious drugs comes the concern of drug safety and drug–drug interactions (DDI). Learning about drug interactions through experience of using DAA will help to avoid drug-related toxicities. Of great concern are the patients infected at a later age because most of these have other comorbid illnesses such as hypertension, diabetes, heart failure, dyslipidemia, or those co-infected with HIV and on antiretroviral drugs. The issue is also important in patients taking immunosuppressive drugs after organ transplants or for inflammatory diseases.
Three mechanisms need to be understood in order to simplify the mechanism of DDI. The first mechanism operates in the blood stream and with protein binding. Displacement of the drug binding to protein can initiate over or underexposure to the active drug. The second mechanism is related to and comes out of cell transportation. Affection of these proteins, polypeptides (1B1 and 1B3) and P-glycoprotein (P-gp), related to influx (drug penetration within cell) and efflux (elimination out of the cell), respectively, are part of drug interaction. The third mechanism is related to liver metabolism itself and drug clearance that affects cytochrome P450 and glucuronidation. This is the most common route for influencing drug metabolism, leading to abnormal drug exposure.
One of the most helpful initiatives has been the creation of a website for DDI, which has been led by the University of Liverpool. Queries on drug interactions can be rapidly solved on this website (www.hep-druginteractions.org). Moreover, it is updated on a regular basis, as new information becomes available, and hence can be considered reliable.
HCV protease inhibitor
SMV and PTV are of the new PI class of DAAs. SMV has a long half-life, and is extensively bound to plasma proteins (>99.9%), primarily to albumin. Elimination occurs via biliary excretion whereas renal excretion is negligible. SMV moderately inhibits CYP3A4 and P-gp in the gut and OATO1B1 in the hepatocyte.[33] Therefore, SMV should not be prescribed with HIV PIs and neither with HIV non-nucleoside analog inhibitors. Tenofovir, emtricitabine, lamivudine, and abacavir have no interactions with SMV, and can thus safely be used in patients receiving this drug. In individuals with impaired liver function, SMV elimination is reduced owing to its primary elimination by the liver, and exposure to SMV increases from 2.4 to 5.2-folds. A number of compounds are contraindicated in patients receiving SMV, including anticonvulsants (carbamazepine, oxcarbazepine, phenobarbital, phenytoin), antibiotics (erythromycin, clarithromycin, telithromycin), antimycobacterials (rifampin, rifabutin, rifapentine), systemically administered antifungals (itraconazole, ketoconazole, posaconazole, fluconazole, voriconazole), and systemically administered dexamethasone and cisapride. Dose adjustments are needed with some antiarrhythmics, warfarin, calcium channel blockers, HMG Co-A reductase inhibitors, and sedative/anxiolytics. No dose changes are required when used in combination with the immunosuppressants tacrolimus and sirolimus; however, monitoring of blood concentrations of the tacrolimus and sirolimus is recommended. In contrast, use of cyclosporine has been shown to result in significantly increased plasma concentrations of SMV (due to hepatic uptake transporter inhibition) such that it is not recommended to coadminister the drugs.
PTV is boosted with ritonavir and both inhibitors of CYP3A4. High exposure to medications that are metabolized by this complex is a major concern.[34] Drug interactions need to be carefully considered in the setting of coinfection with HIV. A number of drugs are contraindicated because elevated plasma exposure would lead to serious adverse events, among which are alfuzosin, amiodarone, astemizole, terfenadine, cisapride, ergot derivatives, lovastatin, simvastatin, atorvastatin, oral midazolam, triazolam, quetiapine, quinidine, salmeterol, and enzyme inducers that might compromise virological efficacy, e.g., carbamazepine, phenytoin, phenobarbital, rifampicin, St John's wort, enzalutamide, and enzyme inhibitors that might increase PTV exposure, e.g., azole antifungals, and some macrolide antibiotics. Tenofovir reduces PTV exposure by 32%. Conversely, tenofovir increases PTV by 24%.
GZR is an HCV NS3/4A PI and a substrate of OATP1B1/3 transporters. The related drug interactions of GZR/EBR combination have been mentioned in the EBR section.
Hepatitis C virus polymerase inhibitors
Hepatitis C virus NS5B polymerase inhibitors
SOF is nucleos (t) ide analog. It requires phosphorylation in the liver to be active as a chain terminator of the nascent HCV RNA chains within the infected hepatocytes. The major form circulating in the blood is the inactive metabolite GS-331007, which is eliminated by the kidney. Thus, SOF exposure increases in patients with renal impairment and dose adjustments should be considered.[35] In cirrhotic patients, SOF exposure increases by 130%. SOF is transported by P-gp and any potent drugs. P-gp inducers significantly decrease SOF plasma concentrations and may lead to a reduced therapeutic effect. Thus, SOF should not be administered with other known inducers of P-gp such as rifampin, carbamazepine, and phenytoin. There are potential interactions that may occur with rifabutin, rifpentine, and modafinil. SOF coadministration with tenofovir along with HIV PIs is discouraged as increased tenofovir disoproxil fumarate exposure enhances the risk of tubulopathy and necessitates periodic checking of glucosuria, phosphaturia, and proteinuria. No significant DDI have been reported with HIV medications. Administration of amiodarone with SOF is contraindicated because of a serious risk of symptomatic bradycardia.
DSV is a non-nucleoside polymerase inhibitor. It is mainly biliary excreted. DSV does not exert inhibitory or inducing effects on CYP450, and therefore, no significant major drug interactions are expected.[36]
Hepatitis C virus NS5A polymerase inhibitors
DCV, LDV, and OBV are of this group with a lower barrier to resistance, with frequent selection of mutations at amino acid residues A30, L31, and Y93.
DCV is absorbed in the intestine and bioavailability is reduced by 23% with a fatty meal. Elimination of DCV is mainly fecal (88%) with a small amount excreted in urine.[37] In contrast, DCV exposure diminishes in patients with hepatic insufficiency, most likely as result of hypoalbuminemia, although the free concentration of the drug does not change much; therefore, no dose adjustment is recommended. DCV is a substrate for CYP3A4 and P-gp, and inhibits transporters organic anion transporting polypeptides 1/3 as well as P-gp. This further explains why HIV PIs boosted with ritonavir increase DCV exposure by two fold. Therefore, the daily dose of DCV must be reduced to half (30 mg/day) when coadministered. DCV slightly increases cyclosporine or tacrolimus exposure. On the other hand, cyclosporine increases DCV concentrations by 40%.
LDV is administered with SOF. It exhibits very low potential for drug interactions with lower potency.[38] LDV is mainly excreted in bile and transported by P-gp and breast cancer resistant protein (BCRP). LDV needs to be monitored closely when used with the statin group. Rosuvastatin is also not recommended. The concentration and solubility of LDV decreases with high pH, therefore, proton pump inhibitors (PPI), antacids, and H2-receptor antagonists are likely to decrease concentrations of LDV. Both H2-receptor antagonists and PPI need to be administered simultaneously or 12 h apart.
Currently, no safety and efficacy data on the combination of SOF and LDV administered along with boosted HIV protease containing regimens have been reported upon.
OBV is a substrate of CYP3A4 and P-gp, and inhibits CYP2C8 and UGT1A1. In patients with moderate-to-severe hepatic insufficiency, OBV exposure increases by up to 55%. It contributes to hyperbilirubinemia when taken with other UGT1A1 substrates.[39]
EBR is combined with GZR, an HCV NS3/4A PI, and both are substrates of CYP3A and P-gp; however, the role of intestinal P-gp in the absorption of EBR and GZR appears to be minimal. EBR/GZR are contraindicated in strong CYP3A inducers (phenytoin, carbamazepine, rifampicin, HIV medications such as atazanavir, darunavir, lopinavir, saquinavir, tipranavir) or inhbitiors (cyclosporine) efavirenz.
EBR/GZR are not recommended with moderate CYP3A inducers (as nafcillin, some HIV drugs, modafinil,) or inhibitors (elvitegravir, cobicistat) because these either decrease or increase the plasma concentration of both drugs, respectively. No dose adjustments are needed when EBR/GZR are used with the following drugs individually: acid reducing agents (proton pump inhibitors, H2 blockers, antacids), buprenorphine/naloxone, digoxin, dolutegravir, methadone, mycophenolate mofetil, oral contraceptive pills, phosphate binders, pitavastatin, pravastatin, prednisone, raltegravir, RBV, rilpivirine, tenofovir disoproxil fumarate, and SOF. No clinically relevant DDI is expected when EBR/GZR are co-administered with abacavir, emtricitabine, entecavir, and lamivudine.[40]
Monitoring during IFN-free regimens
Clinical assessment during treatment with an IFN-free regimen focuses on adherence to the regimen and the emergence of adverse effects.
Monitoring viral levels during treatment with IFN-free regimens has minimal prognostic value because almost all patients without cirrhosis in large clinical trials of IFN-free regimens achieve an undetectable HCV viral level after 4 weeks of treatment.[41]
An additional reason to check viral levels during therapy is to assess adherence to the regimen. Given the expense of the medications and the potential risk of viral resistance with inappropriate use, HCV RNA quantitative testing at week 4 in clinical practice and also rechecking HCV RNA quantitative testing at week 6 if the week 4 level is detectable, and discontinuing therapy if the level has increased by >1 log is recommended.
The clinical value of a week 12 (or end of treatment) viral level is uncertain, and most providers do not routinely check it. It is undetectable in a vast majority of treated patients, even among those who have subsequent viral relapse. In one study, all 6 patients with quantifiable but low level (<65 IU/mL) viremia at the end of DAA-based treatment had achieved an SVR.
Follow-up after treatment
Virological response to treatment should be assessed by checking the viral load at 12 to 24 weeks following the cessation of therapy. SVR is defined by an undetectable viral level at this point. An undetectable level at week 12 after treatment is generally maintained until week 24. However, a small proportion of patients (approximately 2%) experience virological relapse between weeks 12 and 24.[42,43]
Patients who achieve an SVR and do not have bridging fibrosis or cirrhosis do not require any specific follow-up for their HCV, even though some physicians will check an HCV viral load 1 year after the completion of treatment to confirm that the patient has achieved an SVR. On the other hand, those patients who fail to achieve an SVR should be followed for signs of progression of their liver disease.
Patients with bridging fibrosis and cirrhosis, regardless of whether they attain an SVR, warrant ongoing monitoring because they continue to be at a risk of hepatocellular carcinoma or other complications of advanced liver disease, which require ongoing surveillance.
Treatment of hepatitis C virus genotype 1
Treatment of HCV GT1 used to be a challenge, with the least acceptable chance of SVR among other genotypes. However, with the recent advances in direct acting antivirals, the SVR rate for these patients has increased dramatically.[44,45,46]
The choice of therapy here depends on factors such as efficacy, duration, adverse side effects, previous exposure to therapy, type of previous response, and degree of fibrosis.[44,45] DAA-based regimens result in higher SVR rates for GT1 infected patients.[44,45]
GT1a infected patients are more likely to develop resistant variants, including those who previously had high-level resistance leading to virological failure. They tend to have lower response rates and higher relapse rates than patients with HCV GT1b with certain regimens.[47]
Approximately 15% of GT1a patients have NS5A resistance associated variants (RAV) without exposure to NS5A inhibitors. Such patients tend to have a lower treatment response to DAA.[48]
Regimen options
Currently, there are many therapeutic options available. These include IFN-free therapy and IFN-based therapy [Tables 2 and 3].
Table 2.
Treatment recommendations for patients with chronic hepatitis C without cirrhosis, including the treatment-naïve and treatment-experienced
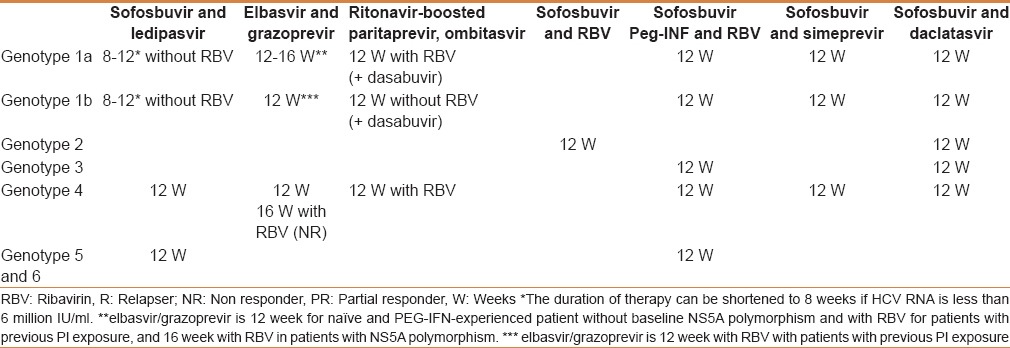
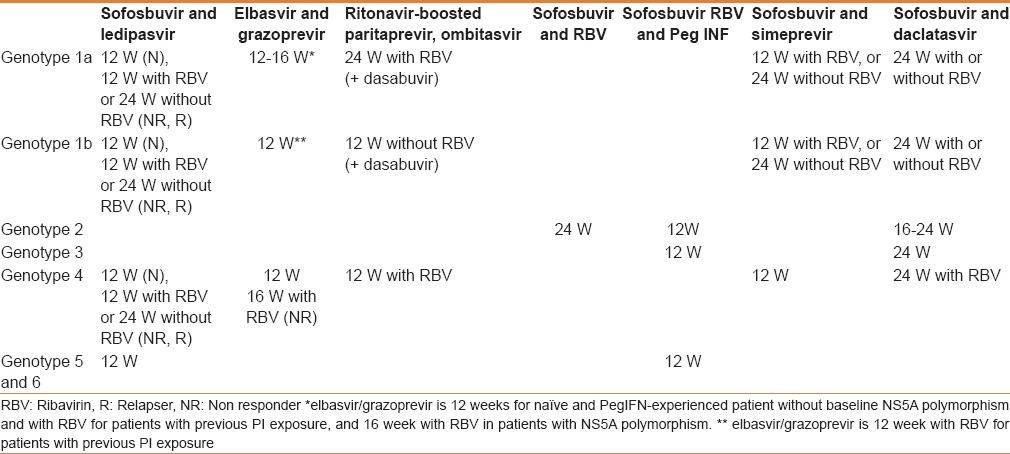
Table 3.
Treatment recommendations for patients with chronic hepatitis C with compensated (Child-Pugh A) cirrhosis, including treatment-naïve and treatment-experienced patients
Interferon-free regimens
For patients with chronic GT1 HCV infection, an IFN-free regimen has become the more popular regimen than the IFN-containing one. Most IFN-free regimens have been shown to be highly effective for all patient populations and are generally well tolerated, without the well-known toxicity associated with IFN. Most clinicians and patients prefer IFN-free regimens.
The regimen of LDV/SOF is a preferred antiviral regimen for the vast majority of patients and clinicians with chronic HCV infection. It is available in a once-daily fixed dose combination tablet of the NS5A inhibitor LDV (90 mg) and the NS5B inhibitor SOF (400 mg) and is highly effective for both treatment-naïve and experienced patients with GT1 infection, even in the setting of cirrhosis.[49,50]
The standard duration of therapy is for 12 weeks for cirrhotic treatment-naïve patients, as well as noncirrhotic and cirrhotic treatment-experienced patients (albeit with the addition of RBV). Eight weeks of therapy appears to be sufficient for noncirrhotic treatment-naïve patients with viral levels <6 million IU/mL.[51] The efficacy of LDV/SOF does not appear to be significantly improved by the addition of RBV.[51,52]
Several evidential studies support the use of LDV/SOF based on data from several clinical trials in both treatment-naïve and experienced patients.[53,54]
In the open-label ION-1 trial, SVR rates among 865 treatment-naïve patients with GT1 infection randomly assigned to receive LDV/SOF with or without weight-based RBV for 12 or 24 weeks ranged from 97 to 99% across all four groups. SVR rates were similarly high among subgroups that have previously been considered difficult to treat. SVR rates ranged from 94 to 100% among patients with cirrhosis. Among those without cirrhosis, an even shorter duration of therapy appears highly effective.
In an open-label ION-3 trial, 647 such patients with GT1 infection were randomly assigned to receive LDV/SOF for 8 or 12 weeks or LDV/SOF with RBV for 8 weeks. SVR rates ranged from 93 to 95 across all groups. Among those with a baseline HCV RNA <6 million IU/mL, SVR rates with LDV/SOF alone were 97 and 96% for 8 and 12 weeks of therapy, respectively. Treatment for fewer than 8 weeks with LDV/SOF appears to be less effective.[55,56,57,58]
In the open-label ION-2 trial, 440 patients with GT1 infection who had failed prior treatment with PegIFN plus RBV, with or without a PI, were randomly assigned to receive a once-daily fixed dose combination tablet of LDV/SOF with or without weight-based RBV for 12 or 24 weeks.[59,60]
SVR rates ranged from 94 to 96% with 12 weeks of therapy (with or without RBV) and were 99% with 24 weeks. Overall, 11 patients had a documented virological relapse after treatment; all the patients had received 12 weeks of therapy. Response rates did not differ by type of prior treatment failure (relapse versus nonresponse) or prior regimen (with versus without a PI). Among the 44 patients with cirrhosis who received LDV/SOF without RBV, SVR rates were improved with 24 weeks, compared to 12 weeks of treatment (100 versus 86%). In a subsequent trial of patients with cirrhosis who had failed PegIFN, RBV, and a PI, treatment with LDV/SOF plus RBV for 12 weeks resulted in similar SVR rates as LDV/SOF plus placebo for 24 weeks (96 and 97%, respectively).[61,62]
This finding was supported by an analysis of the treatment-experienced patients with cirrhosis included in several initial trials of LDV/SOF, which also showed that the two regimens were comparable in this population. Even patients who had relapsed on a prior SOF-containing regimen can be successfully retreated with LDV/SOF, with or without RBV. In a study of 51 patients who had previously failed SOF plus PegIFN and RBV for 12 weeks or SOF with or without RBV for 24 weeks, SVR rates were 98% following 12 weeks of LDV/SOF plus RBV. Similarly, in a study of 14 GT1 infected patients who had relapsed following SOF plus RBV for 24 weeks, all achieved SVR with 12 weeks of LDV/SOF.[58] Patients with stage 3 to 4 fibrosis were well-represented in both the studies. There are no data yet on LDV/SOF as retreatment of patients who previously failed SOF plus SMV.
Another IFN-free regimen for GT1 infection is a combination of the ritonavir-boosted PI PTV (PTV/r) and the NS5A inhibitor OBV (all coformulated in a single tablet) plus the non-nucleotide NS5B inhibitor DSV. It is given with or without weight-based RBV (1000 mg/day if <75 kg or 1200 mg/day if ≥75 kg) for 12 or 24 weeks. It is highly effective in treatment-naïve patients and in those who have failed prior treatment with PegIFN and RBV, even in the setting of cirrhosis. The regimen is particularly effective for subtype 1b infection.[63,64,65,66]
In the absence of cirrhosis, 12 weeks of OBV/PTV/r plus DSV (OBV/PTV/r/DSV) with weight-based RBV results in SVR rates in excess of 95%, regardless of treatment history. In the double-blind SAPPHIRE-I trial, treatment-naïve patients without cirrhosis were randomly assigned to receive 12 weeks of this regimen (n = 473) or placebo (n = 158). The SVR rate was 96% with this regimen, with only one virological breakthrough during treatment and 7 post-treatment relapses. All of the patients who had virological failure had at least one baseline mutation that was associated with resistance to one of the antiviral agents. Similarly, in the SAPPHIRE-II trial of noncirrhotic patients who had failed prior PegIFN plus RBV therapy, 96% of the 297 patients who received the regimen for 12 weeks achieved SVR. Response rates were 95, 100, and 95% among those with prior relapse, prior partial response, and prior null response, respectively.[67,68]
These findings confirm the results of the open label AVIATOR study of patients without cirrhosis, in which 12 weeks of OBV/PTV/r/DSV with weight-based RBV resulted in SVR rates of 96% in treatment-naïve patients and 93% in prior null responders. These outcomes were not different from those after treatment for 24 weeks. However, there were a higher number of virological relapsers following 8 weeks of therapy, suggesting that a shorter duration of treatment is not sufficient.
Among GT1b patients, who are generally more responsive to DAA-based regimens, 12 weeks of OBV/PTV/r/DSV is similarly effective with or without RBV. In the PEARL-III trial of over 400 treatment-naïve, noncirrhotic GT1b infected patients, SVR rates were 99%, regardless of whether RBV was used with this regimen. Similarly, in the PEARL-II trial of 179 treatment-experienced, noncirrhotic GT1b infected patients, the SVR rate was 100% with 12 weeks of OBV/PTV/r/DSV without RBV. In contrast, among over 300 treatment-naïve noncirrhotic GT1a infected patients in the PEARL-IV trial, SVR rates were higher when RBV was included (97 versus 90% without RBV).
High SVR rates can also be achieved with OBV/PTV/r/DSV with weight-based RBV even in the setting of cirrhosis. In the open-label TURQUOISE-II trial, 380 treatment-naïve and experienced patients with cirrhosis were randomly assigned to receive 12 or 24 weeks of this regimen. SVR rates were 92 and 96% for 12 and 24 weeks of treatment, respectively, and the difference between the two was not significant. Overall, more patients in the 12-week treatment arm had documented virological failure (5.9 versus 0.6% with 24 weeks). SVR rates for 12 and 24 weeks of treatment were 89 and 92%, respectively, among patients with subtype 1a infection and were 99 and 100% among those with subtype 1b infection. Subtype 1a infection, a history of prior null response, and former injection drug use were independently associated with failure to achieve SVR.
Among GT1b infected patients with cirrhosis, OBV/PTV/r/DSV remains highly effective, even without the addition of RBV. In an open-label study of 60 such patients with cirrhosis, approximately half of whom had failed prior treatment with PegIFN and RBV, all achieved SVR with 12 weeks of this regimen.[69,70,71]
OBV/PTV/r/DSV with or without RBV is generally well tolerated. In large studies of the combination regimen, adverse events were common, but typically mild. However, in patients with underlying cirrhosis, there have been subsequent reports of hepatic decompensation that occurred within 1–4 weeks of drug initiation, and this regimen should thus be used cautiously in such patients, and should not be used in patients with moderate to severe (Child Pugh B to C) cirrhosis.
EBR/GZR with or without RBV has been approved recently by the Food and Drug Association (FDA) for the treatment of chronic HCV GT1 and GT4 infections in adult patients. The safety and efficacy of EBR/GZR with or without RBV was evaluated in different clinical trials (C-WORTHY and C-EDGE) in patients with GT1 and GT4. The overall SVR rates ranged from 94–97% in GT1, even in patients with cirrhosis.[72,73]
A screening for NS5A polymorphism is important in determining the duration of therapy prior to starting a regimen of EBR/GZR for GT1 patients.[48]
The presence of baseline NS5A RAVs significantly reduces rates of SVR 12 with a 12-week course of the EBR/GZR regimen in GT1a-infected patients. NS5A RAVs were identified at baseline in 12% of GT1a-infected patients enrolled in the C-EDGE study, of which 58% achieved SVR12 compared to an SVR12 rate of 99% in patients without these RAVs receiving 12 weeks of EBR/GZR. Among treatment-naïve patients, the presence of baseline NS5A RAVs with a larger than 5-fold shift to EBR was associated with the most significant reductions in SVR12, with only 22% of GT1a patients, with these high fold change RAVs achieving SVR12.[48]
Recommendations for prolonging the duration of treatment to 16 weeks with inclusion of RBV for treatment-naïve GT1a patients with baseline NS5A RAVs come from the C-EDGE TE trial. In this phase III open-label trial of EBR/GZR, treatment-experienced patients were enrolled. Among 58 GT1a patients who received 16 weeks of therapy with EBR/GZR plus RBV, there were no virologic failures. Subsequent integrated analysis of the EBR/GZR phase 2 and 3 trials have demonstrated SVR 12 rates of 100% (6 of 6 patients) in GT1 patients with pretreatments NS5A RAVs treated with EBR/GZR for 16/18 weeks plus RBV. Based on the known inferior response in patients in the presence of baseline high fold-change NS5A RAVs, NS5A resistance testing is recommended in GT1a patients who are being considered for therapy with EBR/GZR. If baseline high fold-change RAVs are present (polymorphisms at amino acid positions 28, 30, 31, or 93) treatment extension to 16 weeks, with the addition of weight-based RBV (1000 mg [<75 kg] to 1200 mg [≥75 kg]) is recommended to decrease relapse.
The IFN-free combination of the PI SMV (150 mg orally once daily) plus the NS5B inhibitor SOF (400 mg orally once daily) appears effective for the majority of patients with chronic HCV infection, however, the data to support its use are generally more limited than for LDV/SOF and OBV/PTV/r/DSV. The regimen is administered for 12 weeks to those without cirrhosis, and for 24 weeks to those with cirrhosis. No clear benefit is shown with the addition of weight-based RBV (1000 mg/day if <75 kg or 1200 mg/day if ≥75 kg) to SMV plus SOF. Nevertheless, given the overall limited data for this regimen, it is reasonable to add RBV in patients who have characteristics traditionally associated with suboptimal response to antiviral therapy (e.g., cirrhosis, obesity, Black race, unfavorable IL28B genotype) as long as there are no other factors, such as a marginal hemoglobin level, or any renal impairment that could increase the risk of RBV-associated anemia. Given lower response rates, it is not recommended for subtype 1a patients who have cirrhosis and the Q80K viral variant.[74] SMV plus SOF is not an option for patients with a history of treatment failure with a protease inhibitor.[75,76]
In the OPTIMIST-1 trial, 310 GT1-infected patients without cirrhosis were randomly assigned to 12 versus 8 weeks of treatment with SMV plus SOF. Overall SVR rates were greater with 12 weeks of therapy (97 versus 83% with 8 weeks), which resulted in similar outcomes regardless of treatment history (97 and 95% in treatment-naïve and experienced patients, respectively). Among patients with subtype 1a infection, the presence of the Q80K viral variant, which has been associated with decreased response rates to SMV plus PegIFN and RBV, was not associated with variable SVR rates. In contrast, in the OPTIMIST-2 study of 103 patients with cirrhosis, overall SVR rates with 12 weeks of SMV plus SOF were lower at 83% (88 and 79% in treatment-naïve and experienced patients, respectively). In particular, among the 34 subtype 1a-infected patients with the Q80K variant, the SVR rate was only 74% percent.[77,78,79]
Prior limited data had suggested that 24 weeks of therapy might be more effective for patients with cirrhosis. In a pooled analysis of the cohorts in the COSMOS trial, which included 187 patients who were randomly assigned to SMV plus SOF with or without weight-based RBV for 12 or 24 weeks, there was a somewhat greater SVR rate with 24 versus 12 weeks of treatment among patients with cirrhosis (100 versus 86%), however, the numbers were very small. The potential to improve on SVR rates with the 12 week regimen in cirrhotic patients had also been suggested by observational studies, in which SMV/SOF given for 12 weeks resulted in SVR in 75 to 87% in the presence and 88 to 92% in the absence of cirrhosis. Results from the COSMOS trial also suggested that there was no benefit by the addition of RBV; the OPTIMIST studies did not evaluate RBV.
The regimen was well-tolerated in these studies, even among patients with compensated cirrhosis (Child-Pugh class A). The most commonly reported adverse effects were fatigue, headache, and nausea. When observed, anemia and hyperbilirubinemia occurred predominantly in patients who also received RBV. In other studies of SMV containing regimens, photosensitivity and rash have been reported, and patients should thus be cautioned about this risk and instructed to use sun protective measures and limit their exposure to sun. Pharmacologic issues with SMV may limit the use of this regimen. The elimination of SMV is by the liver, and it should not be used in patients with moderate (Child Pugh class B) or severe (Child Pugh class C) hepatic impairment because of two to five-fold increases in exposure.
The combination of the NS5A inhibitor DCV plus the NS5B inhibitor SOF is effective for GT1 infection, although data are limited for patients with cirrhosis. In addition, in the United States, this regimen is not FDA approved for GT1 infection, and hence may not be accessible to many patients.
In an open label trial that included 82 treatment-naïve GT1 infected patients treated with DCV plus SOF for 12 weeks, SVR rates were high (95 and 100% with or without RBV, respectively). Similarly, in a study of HIV and HCV coinfected patients, 12 weeks of DCV plus SOF resulted in SVR rates of 97 and 98% among treatment-naïve (n = 83) and experienced patients (n = 44), respectively. DCV plus SOF for 24 weeks with or without RBV has also been demonstrated to be effective among patients who failed prior therapy with a PI combined with PegIFN and RBV (98% of 42 individuals).[80]
The efficacy is less certain in patients with cirrhosis because of the small number included in these studies. Other data, mainly retrospective or indirect, have suggested that adding weight-based RBV and/or extending treatment to 24 weeks in patients with cirrhosis is associated with acceptably high SVR rates.[81,82]
IFN-containing regimens
In regions where IFN-free regimens are available, IFN-based regimens should not be used for the treatment of GT1 infection. However, they may still be in use in regions that do not have access to newer regimens. The two most-used IFN based therapies are SOF-based and simeprevir-based therapy in addition to RBV. It is effective for treatment-naïve patients and prior relapsers.
SOF plus PegIFN and RBV is effective for treatment-naïve patients and prior relapsers. It has reasonable but lower efficacy for treatment-experienced patients (including those who failed PI-based treatment). SOF, PegIFN, and RBV are initiated together, without a lead-in period. SOF is dosed at 400 mg orally once daily. The three drugs are given for 12 weeks’ duration.[83,84]
The efficacy of SOF plus PegIFN and RBV is greatest among treatment-naïve patients. In an open label trial (NEUTRINO) that included 291 treatment-naïve GT1 HCV-infected patients, 89% of patients achieved SVR12 following 12 weeks of treatment. The SVR12 rates for GT1a and GT1b infected patients were 92 and 82%, respectively. Extending the SOF-containing regimen beyond 12 weeks does not appear to improve efficacy. Patients with cirrhosis tend to have a suboptimal SVR; in the NEUTRINO trial, 80% of the 54 participants with cirrhosis achieved SVR.
The 12-week regimen of SOF, PegIFN, and RBV has not been directly studied in GT1 patients who had previously failed treatment with PegIFN and RBV. However, SVR rates might be extrapolated based on the assumption that SVR rates among treatment-experienced patients would be similar to those observed among patients with multiple negative predictors of SVR. In an analysis of 52 patients who had several negative predictors (bridging fibrosis or cirrhosis, IL28B non-CC genotype, and HCV RNA > 800,000 IU/ml), the SVR rate was 71% following 12 weeks of SOF, PegIFN, and RBV. However, the analysis cannot distinguish rates between relapsers (who generally have better responses to IFN-based therapy) and partial or null responders. In addition, the regimen may have similar efficacy for patients who have previously failed a PI containing regimen. In a small trial of 50 patients who had previously failed treatment with PegIFN and RBV plus an investigational protease inhibitor with or without an additional DAA, the 12-week regimen of SOF plus PegIFN and RBV achieved SVR in 74%. Approximately half of the participants had been treated with more than one prior course of therapy, and the vast majority had at least one baseline mutation associated with antiviral resistance.
SMV -based PegIFN therapy is another option where other options are not available. SMV should not be used for patients with prior failure of first generation PIs. In addition, GT1a patients who have a baseline Q80K mutation should not be treated with this regimen. In comparison to The first generation PIs (boceprevir and telaprevir), SMV has advantages of once daily dosing and lack of additional anemia.
SMV, PegIFN, and RBV are initiated together, without a lead-in period. SMV is given as 150 mg orally once daily.[85,86] Patients should receive all three drugs for 12 weeks. The duration of additional therapy with PegIFN and RBV alone depends on the prior treatment response: (Treatment-naïve patients and prior relapsers—an additional 12 weeks of PegIFN and RBV [thus, 24 weeks of total therapy]; prior partial and null responders—an additional 36 weeks of PegIFN and RBV [thus, 48 weeks of total therapy]).[87]
The efficacy of SMV plus PegIFN and RBV is greatest among treatment-naïve patients and prior relapsers. In two trials (QUEST 1 and 2), 785 treatment-naïve GT1 HCV-infected patients were randomly assigned to receive SMV or placebo in addition to PegIFN and RBV for 12 weeks.[88,89] Patients who received SMV subsequently received an additional 12 weeks of PegIFN and RBV if they achieved a HCV RNA level <25 IU/mL by week 4 and were undetectable by week 12, or an additional 36 weeks if they did not. Patients who received a placebo received an additional 24 weeks of PegIFN and RBV. Overall, 85–91% of patients in the SMV group qualified for the shorter 24 week total course of therapy. SVR12 rates were substantially higher, with SMV compared with the placebo group (80–81 versus 50%). A trial with a similar study design (PROMISE) among GT1 patients who had relapsed following prior PegIFN and RBV therapy demonstrated a similarly high SVR12 rate with SMV (79 versus 37% with placebo).
However, treatment-experienced patients who had prior partial or null response have a lower likelihood of SVR. In a trial (ASPIRE) of 462 patients who had previously failed IFN and RBV therapy, patients were randomly assigned to SMV (100 or 150 mg dose) for 12, 24, or 48 weeks or placebo in addition to 48 weeks of PegIFN and RBV. In patients with prior relapse to PegIFN and RBV, SVR rates were 82–89%. For partial and null responders, the outcomes varied according to subtype. In patients with a prior partial response, SVR rates were 56% (14 of 25 patients) for GT1a and 88% (38 of 43 patients) for GT1b. In patients with prior null response, SVR rates were 42% (11 of 26 patients) for GT1a and 58% (14 of 24 patients) for GT1b. There was no significant change in efficacy with increased duration of SMV therapy.
Patients with cirrhosis also tend to have lower SVR rates. In the QUEST trials, 60% of the 48 patients with cirrhosis achieved SVR. Among treatment-experienced patients with cirrhosis, data have suggested SVR rates of approximately 75–80% for those with prior relapse or partial response and 31% in those with null response. However, it is important to note that these rates are reported from a small numbers of patients.
Recommendations
-
8.
In noncirrhotic, treatment-naïve, and treatment-experienced patients with genotype 1a and 1b, 12 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) therapy is recommended. (grade A1)
-
9.
In compensated cirrhotic, treatment-naïve patients with genotype 1a and 1b, 12 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) therapy is recommended. (grade A1)
-
10.
In compensated cirrhotic, treatment-experienced patients with genotype 1a and 1b, 12 weeks of daily ledipasvir (90 mg) /sofosbuvir (400 mg) therapy with weight-based RBV or 24 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) therapy (in RBV ineligible patients) is recommended. (grade A1)
-
11.
In noncirrhotic, treatment-naïve patients with HCV genotype 1 with baseline HCV RNA < 6 million IU/mL, 8 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) is recommended. (grade A1).
-
12.
In noncirrhotic, treatment-naïve, and treatment-experienced patients with genotype 1a infection, 12 weeks of daily co-formulated paritaprevir (150 mg) /ritonavir (100 mg)/ombitasvir (25 mg) plus twice-daily dosed dasabuvir (250 mg) with weight-based RBV is recommended. (grade A1)
-
13.
In compensated cirrhotic, treatment-naïve and treatment-experienced patients with genotype 1a infection, 24 weeks of daily co-formulated paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) plus twice-daily dosed dasabuvir (250 mg) with weight-based RBV is recommended. (grade A1)
-
14.
In both noncirrhotic and compensated cirrhotic, treatment-naïve and treatment-experienced patients with genotype 1b infection, 12 weeks of daily co-formulated paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) plus twice-daily dosed dasabuvir (250 mg) without RBV is recommended. (grade A1)
-
15.
In both noncirrhotic and compensated cirrhotic, treatment-naïve and treatment-experienced patients with genotype 1a and 1b infection in whom no baseline high fold change NS5A RAVS for elbasvir are detected, 12 weeks of daily combination of elbasvir (50 mg)/grazoprevir (100 mg) is recommended. (grade A1)
-
16.
In both noncirrhotic and compensated cirrhotic, treatment-naïve and treatment-experienced patients with genotype 1a infection and where baseline high fold change NS5A RAVS for elbasvir are detected, 16 weeks of combination of daily elbasvir (50 mg)/grazoprevir (100 mg) with weight based RBV is recommended. (grade B2)
-
17.
In noncirrhotic treatment-naïve and treatment-experienced patients with genotype 1a or 1b, 12 weeks of daily Sofosbuvir (400 mg) and simeprevir (150 mg) without RBV is recommended. (grade A1)
-
18.
In compensated cirrhotic, treatment-naïve, and treatment-experienced patients with genotype 1a or 1b (in whom no Q80K is detected in genotype 1 a), 24 weeks of daily sofosbuvir (400 mg) and simeprevir (150 mg) with or without RBV is recommended. (grade A1)
-
19.
In noncirrhotic patient, treatment-naïve, and treatment-experienced patients with genotype 1a or 1b, 12 weeks of daily sofosbuvir (400 mg) and daclatasvir (60 mg) is recommended. (grade B1)
-
20.
In compensated cirrhotic, treatment-naïve, and treatment-experienced patients with genotype 1a or 1b, 24 weeks of daily sofosbuvir (400 mg) and daclatasvir (60 mg) with or without RBV is recommended. (grade B2)
-
21.
In noncirrhotic (< F3), treatment-naïve patients with genotype 1a or 1b, 12 weeks of daily sofosbuvir (400 mg) and weight-based RBV with weekly peginterferon is an alternative option when interferon-free regimens are constrained (grade B1).
TREATMENT OF HEPATITIS C VIRUS GENOTYPES 2 AND 3
HCV GT2 and GT3 are less prevalent worldwide (9.1 and 30.1%, respectively) with a noticeable variation in distribution within Western countries—North America (GT2, 12.0% and GT3, 10.4%) and Western Europe (GT2, 10.8% and GT3, 24.8%).[90]
The prevalence of GT2 and GT3 in Saudi Arabia is quite low among the other genotypes, being 4.3 and 2.9%, respectively.[3]
Treatment of hepatitis C virus genotype 2
The currently approved treatment of patients with HCV GT2 infection is a combination of SOF and a weight-based dose of RBV for 12 weeks in treatment-naive patients without cirrhosis. In different trials including the FISSION, POSITRON, and VALENCE trials, the SVR 12 with SOF and RBV were 93%, 95%, and 97%, respectively.[91,92,93]
The second option that can be used is the combination of SOF and PegIFN/RBV for 12 weeks for cirrhotic patients, treatment-experienced patients and those who have failed treatment with SOF and RBV. In the LONESTAR-2 Phase IIb study, which included 14 patients with cirrhosis, subjects received 12 weeks of PegIFN/RBV and SOF. The SVR rate was 96%. Among the 592 patients enrolled in the randomized, open-label BOSON trial, 48 patients had GT2 infection. For the patients with GT2 infection, the SVR 12 rates were 87% with the 16-week course of SOF plus RBV, 100% with 24 weeks of SOF plus RBV, and 94% with 12 weeks of PegIFN/RBV and SOF.[94,95]
A combination of SOF and DCV is another option, and can be used in patients intolerant to RBV as well as those with prior PegIFN/RBV treatment failure. In addition, 12 weeks of SOF and DCV in the ALLY-2 study achieved 100% SVR. In another study by Sulkowski et al., the SVR12 was 92% in 26 patients with GT2 [Tables 2 and 3].[80,81] More recently, the combination of EBR (50 mg)/GZR (100 mg) was studied in the C-SCAPE trial, with an efficacy of 80% (30 patients) when RBV is added and 73% (26 patients) without RBV. This combination regimen does not yield optimal results as compared to those in the previously mentioned studies.[40,96]
Recommendations
-
22.
In noncirrhotic, treatment-naïve, and treatment-experienced patients with gentotype 2, 12 weeks of daily sofosbuvir 400 mg and weight-based RBV is recommended. (grade A2)
-
23.
In compensated cirrhotic, treatment-naïve, and treatment-experienced patients with genotype 2, 24 weeks of daily sofosbuvir 400 mg and weight-based RBV is recommended. (grade A2)
-
24.
In noncirrhotic, treatment-naïve, and treatment-experienced patients with genotype 2, 12 weeks of daily sofosbuvir 400 mg and daclatasvir 60 mg is recommended. (grade B1)
-
25.
In compensated cirrhotic, treatment-naïve, and treatment experienced patients with genotype 2, 16-24 weeks of daily sofosbuvir 400 mg and daclatasvir 60 mg in RBV ineligible is recommended. (grade B1)
-
26.
In compensated cirrhotic, treatment-experienced patients with genotype 2, 12 weeks of daily sofosbuvir (400 mg) and weight-based RBV with weekly peginterferon is recommended. (grade B2).
Treatment of hepatitis C virus genotype 3
Chronic HCV GT3-infected patients are difficult to treat with the new DAAs, and are hence considered a special population. Their options are more limited with difficulties in their treatment when compared to other genotypes [Tables 2 and 3].
For naïve or experienced patients without cirrhosis, one option to be considered is to treat such patients with SOF plus DCV for 12 weeks. The SVR12 results according to the ALLY-3 phase III study were 96% without RBV.[97] For either treatment-experienced or naïve cirrhotic patients, the addition of RBV should be considered, and the treatment potentially extended to 24 weeks. The role of RBV, though, is unclear. However, the extension of the treatment to 24 weeks has been shown to be helpful in cirrhotic patients.[98,99]
Another option is a regimen containing SOF and PegIFN IFN with RBV for 12 weeks. This combination has been shown in the LONESTAR phase 2 study and other studies, with SVR12, of 83 and 91% respectively.[94] Limited data is available in using LDV/SOF in GT3. The ELECTRON-II trial in small number of patients with HCV GT3 has shown that the efficacy of using LDV/SOF with RBV achieved 100% in comparison to 64% without RBV.[100] Finally, the combination of EBR/GZR and SOF for 12 weeks in noncirrhotic HCV GT3 patients achieved SVR12 rates of 100%. Moreover, those who received 8 weeks of this regimen and cirrhotic patients treated for 12 weeks achieved SVR12 of 93 and 91%, respectively.[99]
Recommendations
-
27.
In noncirrhotic and compensated cirrhotic, treatment-naïve, and treatment-experienced patients with genotype 3, 12 weeks of daily sofosbuvir 400 mg and weight based RBV and weekly peginterferon for 12 weeks is recommended. (grade A1)
-
28.
In noncirrhotic, treatment-naïve, and treatment-experienced patients with genotype 3, 12 weeks of daily sofosbuvir 400 mg and daclatasvir 60 mg is recommended. (grade A1)
-
29.
In cirrhotic, treatment-naïve, and treatment experienced-patients with genotype 3, 16–24 weeks of daily sofosbuvir 400 mg and daclatasvir 60 mg is recommended. (grade B1).
Treatment of hepatitis C virus genotype 4
Globally, approximately 20% of all hepatitis C infections are caused by GT4. In addition, it is the dominant genotype in the Middle East, Egypt, North Africa, and sub-Saharan Africa.[101]
Available data from the era before DAAs became available, suggest that treatment-naïve GT4 patients who were treated with a 48-week course of pegylated IFN plus RBV had SVR rates ranging from 40–69%,[102] with even lower SVR rates in GT4 patients with cirrhosis (31%).[103] Although the addition of telaprevir or boceprevir to PegIFN and RBV improved SVR rates in patients with GT1, very little data exists with these agents in patients with GT4 infection. HCV GT4 is underrepresented in most of the new HCV DAA studies; nevertheless, the available, but limited data with the new DAAs suggest high response rates [Tables 2 and 3].
IFN-free regimen
In an open-label multicenter phase 2b PEARL-I study, investigators examined the efficacy and safety of an all-oral IFN-free regimen of OBV plus PTV plus ritonavir (OBV/PTV/r), given with or without RBV in 135 noncirrhotic (treatment-naive and treatment-experienced) patients with chronic HCV GT4 infection. In treatment-naïve patients, the SVR12 rates (HCV RNA < 25 IU/mL) were 100% (42/42) in the RBV-containing regimen and 90.9% (40/44) in the RBV-free regimen. No statistically significant differences in SVR12 rates were noted between the treatment-naive groups (mean difference −9.16% [95% CI: −19.61−1.29]; P = 0.086). All treatment-experienced patients achieved SVR12 (49/49; 100% [95% CI: 92.7–100]). In the RBV-free group, 2 (5%) of 42 treatment-naive patients had a virological relapse, and 1 (2%) of 44 had a virological breakthrough; no virological failures were recorded in the RBV-containing regimen.[104]
In a phase 3 AGATE-I trial, treatment-naïve and experienced HCV GT4 patients with cirrhosis were randomized to OBV/PTV/r plus weight-based RBV for 12 weeks and 16 weeks. SVR 12 was 96 and 100%, respectively, and the treatment was well tolerated.[105] AGATE II (Egypt) is an ongoing phase 3, multicenter, open label trial, which enrolled 160 subjects across 5 sites in Egypt. Noncirrhotic patients (n = 100) received OBV/PTV/r once daily (25 mg/150 mg/100 mg) with weight-based RBV for 12 weeks. Cirrhotic subjects (n = 60) were randomized 1:1 to the same regimen for either 12 or 24 weeks (n = 30/arm). Approximately half were treatment-experienced (61% prior nulls, 24% prior relapsers and 15% partial responders). SVR12 was achieved in 94% of patients in noncirrhotics, 97% in cirrhotics who received the combination regimen for 12 weeks, and data is pending for those assigned to the 24 weeks treatment duration.[106]
The efficacy of a combination of once-daily, fixed-dose EBR/GZR for 12 weeks in cirrhotic (22%) and noncirrhotic treatment-naïve adults with HCV GT1, GT4, or GT6 infection was assessed in a phase 3 placebo-controlled trial. The overall SVR12 was achieved in 299 of 316 (95% [95% CI: 92–97%]) of the patients who received the treatment. All HCV GT4 patients, 18/18 (100%) achieved SVR12. The majority of patients were GT1 with relatively few GT4 and GT6 patients.[107]
A pooled analysis of 103 HCV GT4-infected patients including 66 treatment-naive ones who received 12 weeks of EBR/GZR ± RBV, and 37 treatment-experienced patients, including those who had previously failed PegIFN and RBV ± first-generation PI who received 12, 16, or 18 weeks of GZR/EBR ± RBV, was undertaken. Overall 64/66 (97%) treatment-naive (including 6/6 cirrhotic patients) and 32/37 (86%) treatment-experienced GT4 patients achieved SVR12.[108]
In the SYNERGY trial, a single-center, open-label phase 2a trial, 21 treatments-naïve or experienced patients received a single combination tablet of LDV/SOF per day for 12 weeks. SVR12 was achieved in 20 (95%) of 21 patients (95% CI: 76–100), including 7 patients with cirrhosis.[109] This study has been subjected to criticism, among others regarding the use of the Roche assay (with a lower limit of quantification of 43 IU/mL). Since the guidelines recommend the use of an assay with a lower limit of quantification of 25 IU/mL or lower, as such 71% of patients could be said to have achieved the lower limit of quantification of less than 12 IU/mL.[110]
This combination was also evaluated in a small open-label single-arm study which included 44 HCV GT4-infected patients. Of these, 22 patients were treatment-naïve (1/10 with cirrhotic patients was treatment naive). The overall SVR12 rate was 93% (41/44).[111]
The efficacy of LDV/SOF was shown in the ION-4 study, a phase III trial involving HIV/HCV coinfected treatment of naïve and experienced patients of mainly GT1 plus a few with GT4 (8 patients), including compensated cirrhosis patients. All patients received LDV/SOF as a single fixed-dose combination for 12 weeks. SVR12 was achieved in (8/8) 100% of for HCV GT4 patients. The rates of SVR were similar, regardless of the previous treatment or the presence of cirrhosis.[112]
The response rate for a 12 or 24 weeks of a fixed-dose LDV/SOF, once daily, plus RBV in patients with HCV genotypes 1 or 4 and advanced liver disease, including those with decompensated cirrhosis before and after liver transplantation was assessed in a phase 2, open-label study. There were only 5 patients (1%) with GT4. In non-transplant patients, SVR12 was achieved in 86–89% of patients. In transplant recipients, SVR12 was achieved in 96–98% of patients without cirrhosis or with compensated cirrhosis, in 85–88% of patients with moderate hepatic impairment, in 60–75% of patients with severe hepatic impairment, and by all 6 patients with fibrosing cholestatic hepatitis.[113]
In an open-label phase 2 study, a 24-week regimen of SOF and with RBV was shown to be more efficacious than a 12-week regimen in 60 patients with HCV GT4 of Egyptian ancestry. SVR12 was 68% (95% CI: 49–83%) in the 12-week group, and 93% (95% CI: 77–99%) in the 24-week group. In this study, 50% of the study patients were treatment-experienced and 23% had cirrhosis.[114]
Subsequently, in another study, treatment-naïve and treatment-experienced Egyptian patients with GT4 HCV (103 patients) were randomly assigned to receive either 12 or 24 weeks of SOF 400 mg and RBV 1000–1200 mg daily. Approximately half of the patients had received prior HCV treatment and 17% had cirrhosis. SVR12 rates were 90% (46/51) in the 24-weeks group and 77% (40/52) in the 12-weeks group. Patients with cirrhosis at baseline had lower rates of SVR12 (63% 12 weeks, 78% 24 weeks) than those without cirrhosis (80% 12 weeks, 93% 24 weeks).[115]
PHOTON-2 is an open-label, non-randomized, uncontrolled, phase 3 study of HIV/HCV-co-infected patients, including those with compensated cirrhosis. SVR12 for 31 treatment- naïve patients with HCV GT4 infection who received daily SOF plus weight-based RBV for 24 weeks was 84% (26/31).[116]
Few patients were treated with a DCV-based regimen in the registration trials. The ALLY-2 trial included few HCV GT4 patients co-infected with HIV (3/203), who were treatment-naïve (1 patient was treatment-naïve and there were 2 treatment-experienced patients). All those patients achieved SVR 12 (100%) with 12 weeks treatment with DCV plus SOF.[81] In the ALLY 1 trial, SVR 12 was achieved in all (4/4) HCV GT4 patients with advanced cirrhosis (100%) who were treated with DCV/SOF and RBV for 12 weeks.[82] In the European Multicenter Compassionate Use Program, adults with chronic HCV infection who were at high risk for hepatic decompensation or death within 12 months, received open-label DCV/SOF once daily for 24 weeks. The addition of RBV was permitted at the physician's discretion. GT4 patients were 19/482 (4%). Interim analysis showed that the SVR12 rate was 100% (9 of 9 patients; 5 of them received RBV).[117]
In a large real-world GT4 cohort, the French temporary authorization for use (ATU) program, DCV-based regimens were provided to patients with advanced liver disease, or severe extrahepatic manifestations, after liver transplantation recurrence or those waiting for liver or kidney transplantation. Data analyzed for GT4 (n = 215) patients showed that 74% had cirrhosis, 84% were PegIFN and RBV-experienced, and 35% were HIV coinfected. The overall SVR 12 was achieved in 195/215 patients (91%) (90% in cirrhosis). SVR12 was achieved in 30/31 patients (97%) who received DCV/SOF and RBV for 24 weeks and in 7/8 patients (88%) who received this combination for 12 weeks. SVR12 was achieved in 102/110 (93%) of patients who received DCV/SOF for 24 weeks and 53/63 (84%) who received this regimen for 12 weeks.[118]
The OSIRIS trial assessed SMV in combination with SOF in treatment-naïve and experienced HCV GT4 patients (n = 63). The treatment was given for 8–12 weeks in noncirrhotic and 12 weeks in cirrhotic patients. SVR12 rates were 100% for patients treated for the 12 weeks duration and 75% for patients treated for 8 weeks. Out of the 5 patients who relapsed in the 8 weeks arm, all were nonresponders to other therapies with IL28B noncirrhotic genotype.[119] Real-life data of SOF/SMV +/- RBV for 12 weeks in HCV GT4 treatment-naïve and experienced patients with advanced fibrosis or cirrhosis exists. Available results showed overall SVR12 was achieved in 44/47 patients (93.6%), with rates of 93 and 94.4% in patients treated with SOF/SMV alone and SOF/SMV plus RBV, respectively. This SVR12 results were 100% for F3 and 89% for cirrhosis.[120] Lower SVR12 rates (77%) were reported from another single-center, real-life experience in cirrhotic patients with GT1 and GT4 treated with SOF/SMV alone for 24 weeks or with RBV for 12 weeks.[121]
IFN-containing regimen
In a phase III open-label NEUTRINO trial, the SVR12 rate was 90% (95% Cl: 87–93) for treatment with 12 weeks of SOF combined with PegIFN–RBV in 327 treatment-naïve patients with HCV GT1, GT4, GT5, or GT6 (of whom 98% had GT1 or GT4). SVR12 was achieved in 27/28 (96%) with GT4 infection.[122]
Recommendations
-
30.
In noncirrhotic and compensated cirrhotic, treatment-naïve, and treatment-experienced patients with genotype 4 infection, 12 weeks of daily co-formulated paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) with weight-based RBV is recommended. (grade A1)
-
31.
In noncirrhotic and compensated cirrhotic, treatment-naïve with genotype 4 infection, 12 weeks of daily combination of elbasvir (50 mg)/grazoprevir (100 mg) is recommended. (grade B1)
-
32.
uIn noncirrhotic and compensated cirrhotic, treatment-experienced patients with genotype 4 infection, 12 weeks of daily combination of elbasvir (50 mg)/grazoprevir (100 mg) in relapsers and for 16 weeks in null responders is recommended. (grade B2)
-
33.
In noncirrhotic and compensated cirrhotic, treatment-naïve patients with genotype 4 infection, 12 weeks of daily combination of ledipasvir (90 mg)/sofosbuvir (400 mg) is recommended. (grade B1)
-
34.
In noncirrhotic, treatment-experienced patients with genotype 4 infection, 12 weeks of daily combination of ledipasvir (90 mg)/sofosbuvir (400 mg) is recommended. (grade B2)
-
35.
In compensated cirrhotic, treatment-experienced patients with genotype 4 infection, 12 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) with weight-based RBV or 24 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) without RBV is recommended. (grade C2)
-
36.
In noncirrhotic, treatment-naïve or treatment-experienced patients with genotype 4 infection, 12 weeks of daily sofosbuvir (400 mg) and daclatasvir (60 mg) is recommended. (grade B2)
-
37.
In compensated cirrhotic, treatment-naïve or treatment-experienced patients with genotype 4 infection, 24 weeks of daily sofosbuvir (400 mg) and daclatasvir (60 mg) with weight-based RBV is recommended. (grade B2)
-
38.
In noncirrhotic and compensated cirrhotic, treatment-naïve, and treatment-experienced patients with genotype 4 infection, 12 weeks of daily simeprevir (150 mg) and Sofosbuvir (400 mg) is recommended. (grade B1)
-
39.
In noncirrhotic (< F3), treatment-naïve patients with genotype 4 infection, 12 weeks of daily sofosbuvir (400 mg) and weight-based RBV with weekly peginterferon is an alternative option when interferon-free regimens are constrained. (grade B2)
-
40.
In noncirrhotic (< F3), treatment-naïve patients with genotype 4 infection, 12–24 weeks of daily simeprevir (150 mg) and weight-based RBV with weekly peginterferon is an alternative option when interferon-free regimens are constrained. (grade B1).
Treatment of hepatitis C virus genotypes 5 and 6
HCV infection with GT5 and GT6 is quite rare in the Saudi population.[123] Limited data is available in treating these groups of patients and the recommendations are primarily based on extrapolation of experience from other HCV genotypes [Tables 2 and 3]. In the NEUTRINO phase 3 trial on 320 patients with GT1 and GT4, and 7 patients of GT5 and GT6, who received SOF plus PegIFN and weight-based RBV, all achieved an SVR12.[122]
In the ATOMIC study, Kowdley et al. treated 5 patients infected with GT6 with SOF, PegIFN, and weight-based RBV. All of the patients with GT6 were assigned to 24 weeks of therapy and 5 of 5 (100%) achieved an SVR12.[84]
The LDV/SOF combination is another option with limited data in treating HCV GT5 and GT6. In a small, open-label study conducted in France, a 12-week course of LDV/SOF, 39 (95%) of 41 subjects achieved an SVR12. SVR12 was achieved in 20/21 (95%) of the patients who were treatment- naïve and 19/20 (95%) patients who were treatment-experienced. Eight (89%) of 9 patients with cirrhosis achieved SVR12, whereas 31 (97%) of the 32 patients without cirrhosis achieved SVR12.[124]
Recommendations
-
41.
In noncirrhotic and compensated cirrhotic, treatment-naïve, and treatment experienced patients with genotype 5 and genotype 6 infection, 12 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) therapy is recommended. (grade A1)
-
42.
In noncirrhotic and compensated cirrhotic, treatment-naïve, and treatment experienced patients with genotype 5 and genotype 6 infection, 12 weeks of daily sofosbuvir 400 mg and weight-based RBV, and weekly peginterferon is recommended (grade A1).
Retreatment of patients with direct-acting antiviral failures
Baseline RAVs (especially NS5A) are present in approximately 10–15% of treatment-naïve patients, and this may reduce the efficacy of certain DAA treatments for HCV. Hu et al. investigated the global prevalence of pre-existing RAVs to DAAs. Of 1459 full-length HCV sequences, 71% carried at least one dominant resistance variant. Geographically, the highest RAVs occurred in Asia, followed by Africa, Europe, and America in a frequency of 88%, 74%, 69%, and 63%, respectively. The highest RAVs was observed in HCV GT6 sequences, followed by GT2, GT4, GT1, and GT3 in a frequency of 84%, 83%, 78%, 68%, and 50%, respectively. Furthermore, 46%, 31%, and 11% of sequences were RAVs to NS5A inhibitors, NS3 PIs, and their combinations, respectively. In contrast, NS5B nucleos (t) ide inhibitor-based multi-DAA regimens had a low prevalence of RAVs.[125]
The impact of pretreatment NS5A RAVs in patients with HCV GT1 was investigated. A comprehensive analysis using deep sequencing of NS5A from more than 5000 patients from 17 countries found that the prevalence of NS5A RAVs was similar between the different geographic regions. In addition, no significant differences were observed in NS5A RAVs prevalence between patients with different races or ethnicities. The baseline NS5A RAVs are one of the strongest pretreatment predictors of SVR with different regimens in treatment of patients with HCV GT1a infection. It is recommended to test for RAVs before treatment in specific situations. In GT1a patients, a lower SVR rate (72%) was observed in patients with pretreatment high NS5A RAVs conferring high-level (>1000-fold) resistance to NS5A inhibitors. All patients with GT1a who relapsed had pretreatment NS5A RAVs conferring >1000-fold reduced susceptibility to LDV. In the subset of patients who were treated with LDV/SOF for 12 weeks, SVR12 rates were similar in GT1b patients with and without pretreatment NS5A RAVs.[126]
Recent data suggests that NS5A RAVs persist for 96 weeks in the majority of patients who are treated with LDV, an NS5A inhibitor, without SOF.[127]
Jacobson et al. assessed the impact of baseline NS5A RAVs on the efficacy of EBR/GZR against GT1 HCV for 16 weeks versus 12 weeks. The 12-week regimen with no RBV yielded a 99% SVR12 in patients lacking these baseline RAVs. SVR12 rates were lower with EBR (58%) or NS5A class (86%) RAVs versus no RAVs (98%). The impact of such RAVs on efficacy was no longer seen among patients who were given 16 weeks of treatment with EBR/GZR plus RBV.[48] In the ASTRAL trials, there was no impact of baseline NS5A RAVs on the SVR rates in patients with HCV GT2. However, the SVR12 rate was lower in patients with HCV GT3 who had baseline RAVs.[128,129]
In the light of previous failure of any NS5A inhibitors and minimal liver disease, it seems better to defer treatment pending further data. If urgent treatment is needed in such patients, test for NS3 and NS5A RAVs. NS3 and NS5A testing are not required in patients with previous failure of NS3/4A PIs (including SMV, boceprevir, telaprevir) and those with previous failure of NS5B inhibitors (SOF).[130]
NS3 RAVs have low replication efficacy and disappear over 9–18 months. If considering SMV and SOF in treatment of naïve and treatment-experienced patients with both GT1a HCV infection and compensated cirrhosis, it is preferable to ensure there is no Q80K mutation. If the Q80K variant is present, then consider a regimen other than SMV and SOF.[130]
Patients with previous failure of the triple combination of PegIFN, RBV, and either telaprevir or boceprevir should be treated differently. In such patients, a PI such as SMV should be avoided as the response rate with SOF/SMV was 82% (27/33) according to the TRIO Network real-life study.[79]
Failure to achieve SVR on a PI, an NS5A inhibitor or a non-nucleoside inhibitor of HCV polymerase can indicate resistance to the selected drug. Therefore, patients who failed to respond to a DAA-containing therapy should be treated again with a DAA with a high barrier to resistance such as SOF, plus drugs with no cross-resistance with the drugs already administered.[45,62,131,132,133,134,135,136]
Recommendations
The following are the treatment recommendations for retreatment of patients with chronic hepatitis C who have failed to achieve an SVR on prior antiviral therapy containing one or several DAA(s):
-
43.
Patients with genotype 1, who failed treatment with peginterferon, RBV, and NS3 protease inhibitors (telaprevir, boceprevir or simeprevir) can be treated as the following:
- In noncirrhotics, 12 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) therapy is recommended. (grade A1)
- In noncirrhotic, 12 weeks of daily sofosbuvir 400 mg and daclatasvir 60 mg is recommended. (grade B2)
- In noncirrhotic and cirrhotics, 12 weeks of daily combination of elbasvir (50 mg)/grazoprevir (100 mg) with weight-based RBV is recommended. In patients with genotype 1a infection with baseline high fold change NS5A RAVS for elbasvir, 16 weeks of combination of daily elbasvir (50 mg)/grazoprevir (100 mg) with weight-based RBV is recommended. (grade B2)
- In compensated cirrhotics, 12 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) therapy with weight-based RBV or 24 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) therapy in RBV ineligible, is recommended. (grade A1)
- In compensated cirrhotic, 24 weeks of daily sofosbuvir 400 mg and daclatasvir 60 mg with or without weight-based RBV is recommended. (grade B2)
-
44.
Patients who failed treatment of sofosbuvir alone, in combination with RBV, or in combination with peginterferon and RBV can be treated according to the genotypes:
-
Genotype 1:
- In noncirrhotic, 12 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg), sofosbuvir 400 mg and daclatasvir 60 mg, sofosbuvir (400 mg), and simeprevir (150 mg) or co-formulated paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) plus twice-daily dosed DSV (250 mg), all with weight-based RBV is recommended. (grade B2)
- In compensated cirrhotic, 24 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg), sofosbuvir 400 mg and daclatasvir 60 mg, sofosbuvir (400 mg) and simeprevir (150 mg), or co-formulated paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) plus twice-daily dosed dasabuvir (250 mg) all with weight-based RBV is recommended. (grade B2)
-
Genotype 4:
- In noncirrhotic, 12 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg), sofosbuvir 400 mg and daclatasvir 60 mg, sofosbuvir (400 mg) and simeprevir (150 mg), or co-formulated paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) all with weight-based RBV is recommended. (grade C2)
- In compensated cirrhotic, 24 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg), sofosbuvir 400 mg and daclatasvir 60 mg, sofosbuvir (400 mg) and simeprevir (150 mg), or co-formulated paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) all with weight-based RBV is recommended. (grade C2)
-
Genotype 2 or 3:
- In noncirrhotic and compensated cirrhotic, 24 weeks of daily sofosbuvir 400 mg and daclatasvir 60 mg with weight-based RBV is recommended. (grade C2)
- In noncirrhotic and compensated cirrhotic, 12 weeks of daily sofosbuvir (400 mg) and weight-based RBV with weekly peginterferon is recommended. (grade C2)
-
-
45.
Patients with genotype 1 or 4, who failed treatment with sofosbuvir and simeprevir, deferral of treatment is recommended for those who do not have cirrhosis or reason to start treatment urgently, pending availability of data. (grade C2)
Testing for RAVs that decreased susceptibility to NS3 protease inhibitors and to NS5A inhibitors are recommended:
-
Genotype 1:
- In noncirrhotic, 12 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) or sofosbuvir 400 mg and daclatasvir 60 mg all with weight-based RBV is recommended. (grade B2)
- In compensated cirrhotic, 24 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) or sofosbuvir 400 mg and daclatasvir 60 mg all with weight-based RBV is recommended. (grade B2)
-
Genotype 4:
- In noncirrhotic, 12 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) or sofosbuvir 400 mg and daclatasvir 60 mg all with weight-based RBV is recommended. (grade C2)
- In compensated cirrhotic, 24 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) or sofosbuvir 400 mg and daclatasvir 60 mg all with weight-based RBV is recommended. (grade C2)
-
-
46.
Patients, who failed treatment of sofosbuvir and daclatasvir, deferral of treatment is recommended for those who do not have cirrhosis or reason to start treatment urgently, pending availability of data. (grade C2)
Testing for RAVs that decreased susceptibility to NS3 protease inhibitors and to NS5A inhibitors are recommended. When needed, patients can be treated according to genotypes:
- Genotype 1: 24 weeks of daily sofosbuvir 400 mg and simeprevir 150 mg with weight-based RBV is recommended in absence of NS3 RAVs. (grade B2)
- Genotype 4: 24 weeks of daily sofosbuvir 400 mg and simeprevir 150 mg with weight-based RBV is recommended in absence of NS3 RAVs. (grade C2)
- Genotype 2 or 3: In noncirrhotic and in compensated cirrhotic, 12 weeks of daily sofosbuvir (400 mg) and weight-based RBV with weekly peginterferon is recommended. (grade C2)
-
47.
Patients, who failed treatment of sofosbuvir and ledipasvir, deferral of treatment is recommended for those who do not have cirrhosis or reason to start treatment urgently, pending availability of data. (grade C2) Testing for RAVs that decrease susceptibility to NS3 protease inhibitors and to NS5A inhibitors are recommended. When needed, patients can be treated according to genotypes: For genotype 1 or 4:
- Genotype 1: 24 weeks of daily sofosbuvir 400 mg and simeprevir 150 mg with weight-based RBV is recommended in absence of NS3 RAVs. (grade B2)
- Genotype 4: 24 weeks of daily sofosbuvir 400 mg and simeprevir 150 mg with weight-based RBV is recommended in absence of NS3 RAVs. (grade C2)
-
48.
Patients with genotype 1 or 4, who failed treatment of ritonavir-boosted paritaprevir, ombitasvir and dasabuvir, can be treated with:
- In noncirrhotic, 12 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) or sofosbuvir 400 mg and daclatasvir 60 mg, all with weight-based RBV is recommended. (grade C2)
- In compensated cirrhotic, 24 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) or sofosbuvir 400 mg and daclatasvir 60 mg, all with weight-based RBV is recommended. (grade C2)
-
49.
Patients with genotype 4, who failed treatment of ritonavir-boosted paritaprevir and ombitasvir, can be treated with:
- In noncirrhotic, 12 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) or sofosbuvir 400 mg and daclatasvir 60 mg, all with weight-based RBV is recommended. (grade C2)
- In compensated cirrhotic, 24 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) or sofosbuvir 400 mg and daclatasvir 60 mg, all with weight-based RBV is recommended. (grade C2).
Treatment of hepatitis C-related decompensated cirrhosis
Patients are considered to have decompensated cirrhosis if they develop jaundice, variceal bleeding, ascites, or encephalopathy. The immediate treatment goal for patients with decompensated cirrhosis will differ, based on whether the patient is a candidate for liver transplantation. Patients with detectable HCV RNA at the time of liver transplantation will uniformly infect their new liver with HCV, which significantly reduces the life of the liver graft. The main short-term goal of anti-HCV therapy in patients with decompensated cirrhosis who are not candidates for transplantation is to achieve an SVR, anticipating that some degree of liver fibrosis will reverse as a result of therapy and the patient could stabilize and/or improve their clinical condition and chances of survival. For HCV-infected patients who are candidates for liver transplantation, the goal of HCV therapy is to completely suppress HCV RNA prior to transplantation, thus aiming to prevent reinfection of the new liver with HCV and to improve post-transplantation outcomes [Table 4].
Table 4.
Treatment of hepatitis C-related decompensated cirrhosis

Several meta-analyses have shown the relationship between the achievement of SVR and a clear reduction in the risk of HCC.[29,137] However, most of these studies are observational and retrospective and were based on SVR achieved with IFN-based treatments. Because IFN has been shown to improve outcomes following ablation or resection of HCV, it is possible that the high rates of SVR achieved with new IFN-free regimens could reduce the risk of recurrence following resection or ablation of HCC.[138] If the incidence of recurrent HCC can be reduced via this strategy, higher rates of resection or ablation plus an SVR with antiviral treatment could possibly reduce the subsequent need for transplantation for HCV-associated HCC. Further data is required to evaluate the impact of highly effective IFN-free regimens on the risk of recurrent HCC following resection or ablation.
It might be argued that because the treatment of HCV infection can be achieved in the vast majority of patients after transplantation, there is no need to treat HCV infection prior to transplantation, especially because the duration of antiviral therapy cannot be predicted in a patient on the waiting list. Nevertheless, prevention of liver graft infection substantially facilitates post-transplant management. In addition, improvement of liver function implies delisting of some patients,[139] an appropriate strategy in the current context of organ shortage.[140] In addition, the risk of HCC recurrence could theoretically be reduced by antiviral therapy after resection; thus, more patients could possibly be offered resection.
Patients with hepatocellular carcinoma or decompensated cirrhosis with or without an indication for liver transplantation
A 48-week regimen of SOF and RBV is being assessed in patients with cirrhosis and portal hypertension.[141] Preliminary results have demonstrated excellent on treatment responses and even slight improvements in liver function tests. However, the long-term clinical benefits and the effect of this treatment on portal pressure have not been reported. The SOLAR-2 study was a multicenter randomized controlled trial of 108 patients with HCV GT1 and GT4 who had decompensated cirrhosis (Child-Pugh score up to 12). The study assessed the safety and efficacy of the fixed-dose combination of LDV/SOF with RBV (initial dose of 600 mg, increased as tolerated) for 12 or 24 weeks.[140] Most patients who started RBV at 600 mg per day did not receive higher doses. All participants had a hemoglobin level greater than 10 g/dL and a creatinine clearance (CrCl) rate greater than 40 mL/min. The SVR rates were 87% (45/52) and 89% (42/47) after 12 and 24 weeks of treatment, respectively; treatment was equally effective in patients with Child-Pugh B and Child-Pugh C cirrhosis. Moreover, there was a clear beneficial effect of viral clearance on liver function, with improvements in bilirubin, albumin, and INR values. Baseline Child-Pugh and Model for End-Stage Liver Disease (MELD) scores improved in more than 50% of the treated patients, however, some patients did have worsening hepatic function. During the course of the study, 5 (5%) patients died from various causes, but none of the deaths were attributed to antiviral therapy. Grade 3 or 4 adverse events were more common in the 24-week arm (34%) than those in the 12-week arm (15%). These preliminary results indicate that a 12-week course of LDV/SOF and RBV is an appropriate regimen for patients with decompensated cirrhosis who are infected with HCV GT1 or GT4, and that patients with decompensated cirrhosis benefit from this treatment regimen. Although the study was not specifically designed to assess the impact of antiviral therapy in patients awaiting liver transplantation, the data support the use of this combination in patients with compensated and decompensated cirrhosis who are on the transplant waiting list. The treatment indication should take into account the presence of comorbidities that may impact survival. Moreover, it will be important to assess the benefit of HCV elimination on liver function and subsequent survival at later time points. Data in patients with more advanced liver disease (Child-Pugh >12) are limited.
A multicenter, double-blind study from France reported the use of daily LDV/SOF for 24 weeks compared with daily LDV/SOF and RBV for 12 weeks in 154 patients with compensated cirrhosis and HCV GT1 infection in whom prior PegIFN and RBV treatment had failed (for most, treatment with PegIFN, RBV, and a PI had also failed).[55] The SVR12 rates were 96% with the 12-week regimen and 97% with the 24-week regimen. In the light of these results, and by extrapolating the data to include only decompensated cirrhosis patients, it is reasonable to consider daily LDV/SOF and RBV for 12 weeks in patients with decompensated cirrhosis in whom prior SOF-based treatment has failed.
DCV has been used in a number of oral regimens for patients with decompensated cirrhosis. In the phase III ALLY-1 study,[82] DCV (60 mg daily) was administered in combination with daily SOF (400 mg) and a low initial dose of RBV (600 mg) for 12 weeks to treatment-naïve and experienced patients who predominantly had HCV GT1 infection, and in 2 specific populations, that is, those with advanced cirrhosis (Child Pugh class B and C; n = 60) and those with recurrent HCV infection post-transplant (n = 53). The SVR12 rate was 83% among those with advanced cirrhosis and 94% among those with recurrent HCV infection post-transplant. In the population with advanced cirrhosis, SVR12 rate was 76% among patients with HCV GT1a and 100% among patients with HCV GT1b. In the population with advanced cirrhosis, the SVR12 rate was 94% among patients with Child Pugh class B cirrhosis and 56% among patients with Child Pugh class C cirrhosis. Among patients with HCV GT3, SVR12 rates were 83 and 91%, respectively, in those with advanced cirrhosis and recurrent post-transplant HCV infection.
The European DCV compassionate-use program utilized the combination of daily DCV and SOF for 24 weeks, with or without RBV in patients with cirrhosis. The interim SVR12 rates of the HCV/HIV coinfected, decompensated cirrhosis (Child Pugh B and C) cohort comprising all genotypes (n = 28) was 88 and 80% in those treated with and without RBV, respectively.[142] Another cohort from the Program of HCV GT3 patients (n = 45) reported SVR12 rates of 86% (7 of 8 patients) and 80% (12 of 15 patients) in Child Pugh B patients among those who received and did not receive RBV, respectively; and SVR12 rates of 100% (2 of 2 patients) and 75% (6 of 8 patients) in Child Pugh C patients with and without RBV, respectively.[143]
Patients with an indication for liver transplantation
In a recently published study,[144] 61 patients infected with GT1 or GT4 with Child Pugh A cirrhosis were treated with SOF and RBV up to 48 weeks prior to transplantation; 46 of them were transplanted. Seventeen patients had Child Pugh scores of 7 or 8 (Child Pugh class B cirrhosis), 45 (73%) patients had HCV GT1, 8 (13%) had HCV GT2, and 7 (11%) had HCV GT3. The per-protocol efficacy population consisted of 43 patients with an HCV RNA level <25 IU/ml at the time of transplantation. Among them, 30 (70%) had post-transplantation SVR12. The duration of undetectable HCV RNA pretransplant was the best predictor of response (undetectable HCV RNA for more than 30 continuous days). This proof of concept study demonstrated that an IFN-free regimen administered for a few weeks before transplantation prevented HCV graft infection in the majority of treated patients. In patients infected with GT2, the combination of SOF and RBV is the treatment of choice, with very high SVR rates. For other genotypes, this combination should only be administered until liver transplantation, if no other treatment choice is available.
In a SOF compassionate-use program for patients with severe recurrent HCV infection following liver transplantation who were predicted to have a less than 6-month survival rate,[87] 78 patients were treated; 44 patients were treated with SOF plus RBV, and 32 patients also received PegIFN. After week 12 of treatment, 91% of patients treated with SOF plus RBV and 75% of those treated with the addition of PegIFN achieved HCV RNA levels below the lower limit of quantification. Of 27 patients evaluated at 12 weeks post-treatment, 15 patients (56%) achieved SVR. Overall, 75% of patients had improved or stable clinical liver disease, including improved hyperbilirubinemia and coagulopathy and a decreased model for end-stage liver disease (MELD) score. In this very sick population, 8 patients died, but most deaths were caused by liver disease progression.
Treatment with PegIFN, RBV, and SOF for 12 weeks is acceptable in patients with compensated (Child-Pugh A) cirrhosis awaiting liver transplantation if IFN-free combinations are not available. This is based on the interim analysis of a study in 164 GT1-infected patients, half treatment-experienced and one-third with cirrhosis, who achieved SVR4 in 85% of cases.[145]
Safety and efficacy data of the combination of ritonavir-boosted PTV, OBV with RBV in compensated cirrhotic patients infected with GT4, and additionally with DSV in GT1 have been reported.[66] In GT1 patients with a platelet count <100,000 cells/mL, the SVR12 rates were 89% and 97% in the 12 and 24-week treatment duration arms, respectively. The SVR rates in patients with an albumin level <35 g/dl were 84 and 89%, respectively. In GT4 infected patients, preliminary data revealed SVR12 rates of 96 and 100% in 12 and 16-week durations, respectively. Thus, this drug combination can be considered in individuals with GT1 and GT4 compensated cirrhosis and HCC who are on the waiting list.
The combination of SOF and SMV, with or without RBV, has been assessed in large real-life cohorts, including a significant number of patients with cirrhosis.[145] In patients with HCV GT1 infection and compensated cirrhosis, the SVR4 rates were in the order of 90%. Preliminary data in 81 GT1-infected patients with decompensated cirrhosis showed an SVR4 rate of 75%, with a good safety profile. In another real-life cohort, the same combination of SOF and SMV, with (n = 117) or without RBV (n = 34), was assessed with HCV GT1 compensated cirrhosis patients with MELD score of >10. Preliminary data with this combination showed SVR12 rates of 66 and 74% with or without RBV, respectively.[146] However, SMV is generally not indicated in patients with decompensated cirrhosis because of the higher drug concentrations observed.
Although there are currently no data regarding the use of LDV/SOF in patients with decompensated cirrhosis and HCV GT3, this regimen may be of value if proven safe and effective.
PegIFN should not be given to patients with decompensated cirrhosis (moderate or severe hepatic impairment; Child Pugh class B or C) because of the potential for worsening hepatic decompensation. Neither telaprevir nor boceprevir should be used for this population because they must be coadministered with PegIFN and RBV. Very minimal data exist for the use of SMV in patients with decompensated cirrhosis, and until additional data becomes available, it should not be used in patients with decompensated cirrhosis.
Recommendations
-
50.
Patients with HCV-induced decompensated cirrhosis (moderate or severe hepatic impairment; Child Pugh class B or C, up to 12 points) should be referred to a medical practitioner with expertise in that condition (ideally in a liver transplant center). (grade C1)
-
51.
Although the long-term benefit of antiviral therapy to reduce the risk of HCC in patients undergoing resection or ablation for HCV-associated HCC is unknown, these patients almost always have advanced fibrosis and should receive appropriate antiviral therapy for their liver disease (grade B2)
-
52.
In patients awaiting liver transplantation, antiviral therapy is indicated because it prevents graft infection (grade A1)
-
53.
Treatment should be initiated as soon as possible in order to complete a full treatment course before transplantation and assess the effect of viral clearance on liver function because significant improvement in liver function may lead to delisting of selected cases (grade B1)
-
54.
The optimal timing of treatment (i.e., before transplantation or post-transplantation) to maximize survival is still debatable and requires individual assessment (grade B2)
-
55.
Due to the limited amount of safety data reported in patients with decompensated cirrhosis awaiting liver transplantation, frequent clinical and laboratory assessment is necessary (grade B2)
-
56.
Patients with HCV genotype 1–6 and decompensated cirrhosis (Child Pugh B and C, up to 12 points), 12 weeks of daily sofosbuvir (400 mg) and daclatasvir (60 mg) and low initial dose of RBV (600 mg, increased as tolerated) is recommended. (grade A2)
-
57.
Patients with HCV genotype 1 or 4, 5, and 6 and decompensated cirrhosis, 12 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) therapy and low initial dose of RBV (600 mg, increased as tolerated) is recommended. (grade B2)
-
58.
Patients with HCV genotype 2 and decompensated cirrhosis, 24 weeks of daily sofosbuvir (400 mg) and low initial dose of RBV (600 mg, increased as tolerated) is recommended. (grade B2)
-
59.
Patients with HCV genotype 1, 4, 5, or 6 and decompensated cirrhosis with contraindications to the use of or with poor tolerance to RBV 24 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) therapy is recommended. (grade B1)
-
60.
All HCV genotypes patients and decompensated cirrhosis with contraindications to the use of or with poor tolerance to RBV 24 weeks of daily sofosbuvir (400 mg) and daclatasvir (60 mg) is recommended. (grade B1)
-
61.
Patients with conserved liver function (Child Pugh A) in whom the indication for transplantation is HCC can be treated according to genotypes. Please see appropriate sections for cirrhotic patients under different genotypes. (grades A1–B2)
-
62.
Patients with conserved liver function (Child Pugh A) in whom the indication for transplantation is HCC can be treated with 12 weeks of daily sofosbuvir (400 mg) and weight-based RBV with weekly peginterferon in patients awaiting liver transplantation if interferon-free combinations are not available. (grade B2).
Treatment of hepatitis C virus post liver transplant
Worldwide, HCV cirrhosis is still the most common indication for liver transplantation, as it is in Saudi Arabia.[147] HCV recurrence in the allograft occurs in more than 95% of patients.[148,149]
Post liver transplant treatment of HCV is a priority given the accelerated progression to fibrosis and graft loss due to immunosuppression.[150,151] Treatment should be initiated as soon as possible after the transplant without depending on the fibrosis score, as was the case in the IFN era, because of the high efficacy of the new DAA with minimal side effects and most of them with no drug interaction with calcineurin inhibitors (CNI) [Table 5].[152] In the SOLAR-1 study, 223 liver transplant recipients were randomly assigned to receive fixed-dose combination LDV (90 mg) and SOF (400 mg) and weight-based RBV (1000 mg [<75 kg] to 1200 mg [>75 kg]) for either 12 weeks or 24 weeks. Subjects were 111 patients with Metavir fibrosis stage F0–F3; 51 patients with HCV GT1 or GT4 and compensated Child-Pugh class A cirrhosis; 61 patients with decompensated Child-Pugh class B or C cirrhosis. SVR was achieved in 96% of patients with fibrosis stages F0–F3 and in 96% of those with compensated cirrhosis, in both the 12 and 24-week arms. For patients with Child Pugh class B or C cirrhosis, RBV was initiated at 600 mg daily followed by dose escalation as tolerated. Only 2% of patients discontinued treatment because of adverse events. Efficacy was lower in patients with Child Pugh class B cirrhosis (85% SVR12) or Child Pugh class C cirrhosis (60% SVR12), with no increase in SVR observed in patients who received 24 weeks of treatment. Mortality rate was 10% during the study among patients with Child Pugh class B or C cirrhosis.[56] In the ALLY-1 study, DCV in combination with daily SOF and RBV (initial dose, 600 mg) was used for 12 weeks in treatment-naïve and experienced patients of predominantly HCV GT1 infection. In 53 patients with recurrent HCV infection post-transplant, the SVR12 was 94%. Among subjects with HCV GT3, post liver transplant the SVR12 was 91%.[82]
Table 5.
Noncirrhotic and compensated cirrhotic chronic HCV post liver transplant
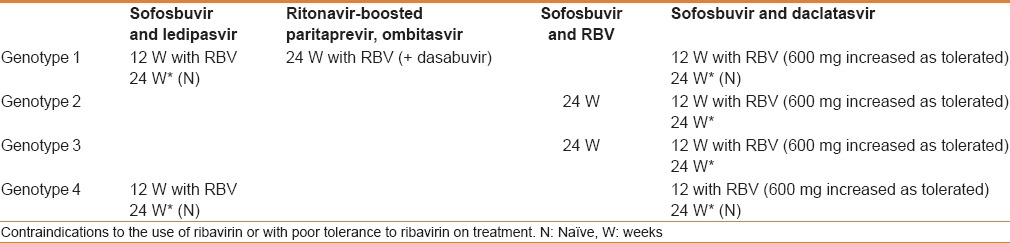
LDV/SOF and DCV do not have any DDI that require the need for any dose adjustment in CNI, rapamycin (mTOR inhibitor), steroid, or mycophenolate levels. On the other hand, OBV/PTV/r/DSV and weight-based RBV given to HCV GT1 post liver transplant[153] has a major drug interaction with CNI. Prospective dose adjustments were needed for cyclosporine and tacrolimus. Interactions between ritonavir and other medications commonly taken by liver transplant recipients are also possible and will require detailed consideration when using this regimen. The efficacy and tolerability of this regimen in patients with more advanced HCV infection post-liver transplant are unknown. In addition, coadministration of single-dose cyclosporine with SMV resulted in a 19% increase in cyclosporine concentrations and no change in SMV concentrations,[154] however, the co-administration of single-dose tacrolimus with SMV did not result in a notable change of tacrolimus concentrations with a 2-fold increase in plasma concentrations of SMV compared with historical data that are unlikely to be clinically significant.
Recommendations
Recommended regimens are listed in groups by level of evidence.
Genotype 1 and 4:
-
63.
In both noncirrhotic and compensated cirrhotic, treatment-naïve and treatment experienced patients with HCV genotype 1 or 4 infection post-transplant:
- 12 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) therapy with weight-based RBV is recommended. (grade A1)
- 12 weeks of daily sofosbuvir (400 mg) and daclatasvir (60 mg) with low initial dose of RBV daily (600 mg, increased as tolerated) is recommended. (grade B1)
-
64.
In both noncirrhotic and compensated cirrhotic, treatment-naïve patients with HCV genotype 1 or 4 infection post-transplant, who are RBV ineligible:
- 24 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) is recommended. (grade B1)
- 24 weeks of daily sofosbuvir (400 mg) and daclatasvir (60 mg) is recommended. (grade B2)
-
65.
In decompensated cirrhotic (Child Pugh class B or C), treatment-naïve and treatment-experienced with HCV genotype 1 or 4 infection post-transplant, 12 weeks of daily ledipasvir (90 mg)/sofosbuvir (400 mg) with low initial dose of RBV (600 mg, increased as tolerated) is recommended. (grade B1)
-
66.
In non-cirrhotic (Metavir F0-F2) with HCV genotype 1 infection post-transplant, alternative treatment can be used (with appropriate dose adjustment of CNIs), 24 weeks of daily co-formulated paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) plus twice-daily dosed dasabuvir (250 mg) with weight-based RBV is recommended. (grade B1).
Genotype 2:
-
67.
In both noncirrhotic and compensated cirrhotic, treatment-naïve and treatment experienced patients with HCV genotype 2 infection post-transplant:
- 12 weeks of daily sofosbuvir (400 mg) and daclatasvir (60 mg), with a low initial dose of RBV (600 mg, increased as tolerated) is recommended. (grade A2)
- 24 weeks of daily sofosbuvir (400 mg) and weight-based RBV is recommended. (grade C2)
-
68.
In both noncirrhotic and compensated cirrhotic, treatment-naïve and treatment experienced patients with HCV genotype 2 infection post-transplant, who are RBV ineligible, 24 weeks of daily sofosbuvir (400 mg) and daclatasvir (60 mg), is recommended. (grade C2)
-
69.
in decompensated cirrhotic (Child-Pugh class B or C), treatment-naïve and treatment experienced with HCV genotype 2, post-transplant, 24 weeks of daily sofosbuvir (400 mg) and RBV (initial dose 600 mg/day, increased monthly by 200 mg/day as tolerated to weight-based dose) is recommended. (grade C2).
Genotype 3:
-
70.
In both noncirrhotic and compensated cirrhotic, treatment-naïve and treatment experienced patients with HCV genotype 3 infection post-transplant, 12 weeks of daily sofosbuvir (400 mg) and daclatasvir (60 mg), with low initial dose of RBV (600 mg, increased as tolerated) is recommended. (grade A2)
-
71.
In both noncirrhotic and compensated cirrhotic, treatment-naïve and treatment experienced patients with HCV genotype 3 infection post-transplant, who are RBV ineligible, 24 weeks of daily sofosbuvir (400 mg) and daclatasvir (60 mg) is recommended. (grade C2)
-
72.
In both non-cirrhotic and compensated cirrhotic, treatment-naïve and treatment-experienced post-transplant patients with HCV genotype 3 infection, alternatively 24 weeks of daily sofosbuvir (400 mg) and weight-based RBV is recommended. (grade B2).
TREATMENT OF SPECIAL GROUPS
Human immunodeficiency virus coinfected patient
Approximately 25% of HIV-infected patients in the West have chronic HCV infection; however, there is no clear data reflecting the prevalence in Saudi Arabian patients. Presence of HIV infection leads to a higher HCV RNA and a lower response rate to PegIFN and RBV treatment.[155]
Coadministration of RBV with didanosine should be avoided to prevent mitochondrial toxicity and fatal lactic acidosis. Progression of liver disease is accelerated in patients with HIV–HCV coinfection,[156] in particular those with a low CD4-positive cell count and impaired immune function.
So far, the response to new DAAs treatment has shown no difference in response between mono-infected or coinfected persons.[157]
The main issue with the new DAA treatment and tenofovir is nephrotoxicity with the use of ritonavir-based therapy. Thus, with the use of OBV/PTV/r/DSV, additional ritonavir should be discontinued, and then restarted when the HCV treatment is completed.
Rilpivirine and efavirenz should not be used with the OBV/PTV/r/DSV regimen because it increases the chance for adverse events such as ALT elevation and neurological side effects.[158]
More data on safety and efficacy in GT1 or GT4 (treatment-naïve and experienced, with or without cirrhosis) been demonstrated with the 12 weeks use of LDV/SOF in HIV-infected patients using antiretrovirals[159] as well as on OBV/PTV/r/DSV with or without RBV.[160] For GT2 or GT3, the recommendations are the same as those for patients without HIV infection.[116]
Combinations of tenofovir and emtricitabine with efavirenz, raltegravir, ritonavir-boosted atazanavir, ritonavir-boosted darunavir, or rilpivirine are all acceptable and well-tolerated with SVR12 rates for GT2 at 89% and GT3 at 84%.
Recommendations
-
73.
HCV with and without HIV coinfection should be treated and retreated in the same manner, however, special attention should be paid to drug–drug interactions. (grade B1)
-
74.
No interruption to HIV treatment is recommended (grade A2), however, pretreatment assessment and consultation with an HIV specialist is highly recommended. (grade A1)
-
75.
Avoid using ledipasvir and tenofovir in patients with CrCl < 60 mL/min; the effect is potentiated when ritonavir is used concomitantly. (grade C2)
-
76.
Ledipasvir/sofosbuvir should not be used with cobicistat and elvitegravir (grade C2) or with tipranavir. (grade B2)
-
77.
Paritaprevir/ritonavir/ombitasvir and dasabuvir should not be used with efavirenz, rilpivirine, darunavir, or ritonavir-boosted lopinavir and in individuals who are not on antiretroviral therapy. (grade B2)
-
78.
Paritaprevir/ritonavir/ombitasvir and dasabuvir can be used with raltegravir (and probably dolutegravir), enfuvirtide, tenofovir, emtricitabine, lamivudine, and atazanavir. The ritonavir dose may need to be adjusted (or held) and then restored when HCV treatment is completed. Both HIV and HCV Medications should be administered at the same time. (grade C2)
-
79.
Simeprevir should not be used with a protease inhibitor or efavirenz, etravirine, nevirapine, cobicistat, however, it can be used with raltegravir (and probably dolutegravir), rilpivirine, maraviroc, enfuvirtide, tenofovir, emtricitabine, lamivudine, and abacavir. (grade B2)
-
80.
RBV should not be used with didanosine, stavudine, or zidovudine. (grade B2).
Hepatitis B virus coinfection
Coinfection with HBV and HCV can often be seen in high-risk patients. The HBV DNA level, although it is often low, may fluctuate, however, HCV is usually the main driver of chronic hepatitis activity. Replicative activity including hepatitis delta (HDV) infection needs to be assessed carefully. When HCV activity is documented, treatment in the same manner as that for mono-infected patient should be considered, despite the potential risk of HBV reactivation.[161] When treatment becomes indicated for HBV, the eGFR should be monitored frequently if simeprevir and tenofovir are used concomitantly because the tenofovir dose might need adjustment.
Recommendations
-
81.
No difference in treatment between mono or coinfected patients. (grade B1)
-
82.
Concurrent HBV nucleoside/nucleotide analogue therapy is indicated once HBV replication is detected (grade B1).
Patients with renal impairment on hemodialysis and renal transplant recipients
Chronic kidney disease (CKD) represents a global health problem. Chronic HCV infection has a significant impact on morbidity and mortality because it may cause deterioration of renal function. The prevalence rates reported in hemodialysis patients in Middle Eastern countries are 68% in Saudi Arabia, with a range of 14.5–94.7%, 26% in Oman, and 80% in Egypt.[162] CKD is common in Saudi Arabia but vastly reduced rates of infection have been seen over past 2 decades. Liver biopsy or transient elastography may be needed before treating these patients because of the discrepancy between the level of the ALT and the extent of histologic damage noted in such patients.[163]
Mild to moderate renal impairment (CrCl rate 30–80 mL/min) is becoming a less serious issue and the new DAAs can be regarded as safe to use. Severe renal impairment (CrCl < 30 mL/min) requires more attention. In severe renal impairment, current clinical trials are limited to GT1 patients. Studies of OBV/PTV/r/DSV with or without RBV (for HCV GT1a or GT1b) in treatment-naive patients without cirrhosis showed promising efficacy with SVR12 achieved in 19/20 (95%), however, RBV-induced anemia occurred frequently.[164] RBV should not be given if the baseline hemoglobin level is less than 10 g/dL.
RBV is poorly tolerated in patients with severe renal impairment and the dose or frequency needs to be reduced and individualized. RBV dosing of 200 mg/day or every other day or thrice weekly after hemodialysis is recommended. DCV, EBR/GZR, LDV, OBV/PTV/r/DSV, and SMV are minimally excreted by the renal site.
More recently, in HCV GT1 treatment-naïïve and experienced patients with CKD stages 4/5 (eGFR < 30 mL/min/1.73 m2, including 75% of patients who were on hemodialysis with small numbers of compensated cirrhotics), EBR/GZR versus placebo was evaluated for a duration of 12 weeks. The SVR12 was up to 99%. The safety profile showed no difference regarding anemia or other adverse events compared to the placebo. Viral relapse was observed in GT1b but there was none in subtype 1a patients.[165]
SOF is excreted via the renal route and is currently undergoing an ongoing prospective observational cohort evaluating its use in mild to severe renal impairment. So far, SVR12 has been similar across the groups, regardless of renal function with higher adverse events profile.
Patients on hemodialysis or post renal transplant patients carry a high prevalence of HCV infection that can affect mortality, although the main cause of death is due to cardiac disease.[166] IFN-based therapy may lead to post renal transplant graft rejection. It mandates an urgent need to treat those patients with IFN-free regimens. Patients on hemodialysis, because of other comorbidities, should be treated with DAAs. In renal transplant recipients, although liver disease has little impact on these patients, using immunosuppression may accelerate the process of liver damage as well as graft function. Moreover, because survival could be affected, treatment for HCV should be considered with intereferon-free regimens with an expectation of cure even for those patients undergoing simultaneous liver-kidney transplantation.[166,167]
Recommendations
-
83.
HCV infection is an urgent and high-priority indication for antiviral therapy and no dose adjustment is required when treating or retreating patient with new DAAs and with mild to moderate renal impairment (CrCl rate >30–80 mL/min). (grade A1)
-
84.
Liver biopsy or transient elastography is important to stage the disease for HCV infected patients on a renal transplant list. (grade C1)
-
85.
Renal transplant candidates should be a high priority for treatment. (grade B1)
-
86.
Elbasvir, grazoprevir, simeprevir, daclatasvir, and paritaprevir/ritonavir/ombitasvir and dasabuvir regimens can be used in patients with severe renal disease. (grade A1)
-
87.
Sofosbuvir should not be used in patients with CrCl below 30 mL/min or in hemodialysis patients until more data is available. (grade B2)
-
88.
Interferon-free, if possible RBV-free, treatment in hemodialysis patients (12 weeks in non-cirrhotic and for 24 weeks in cirrhotic). (grade B1)
-
89.
Non-hepatic solid organ transplant patients should receive interferon-free regimen with special attention to drug–drug interactions. (grade B2)
-
90.
Sofosbuvir, daclatasvir, ledipasvir/sofosbuvir, paritaprevir/ritonavir/ombitasvir with dasabuvir regimens (or without dasabuvir for HCV genotype 4 infection), simeprevir or elbasvir/grazoprevir can be used to treat or retreat HCV infection according to genotypes for patients with mild to moderate renal dysfunction with creatinine clearance more than 30 mL/min with no dose adjustment. (grade A1)
-
91.
Patients with genotype 1a, or 1b, or 4 infection and CrCl below 30 mL/min for whom the urgency to treat is high and kidney transplant is not an immediate option, 12 weeks of daily elbasvir (50 mg)/grazoprevir (100 mg) is recommended. (grade B2)
-
92.
Patients with genotype 1b, and CrCl below 30 mL/min for whom the urgency to treat is high and kidney transplant is not an immediate option, 12 weeks of daily co-formulated paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) plus twice-daily dosed dasabuvir (250 mg) is recommended. (grade B2)
-
93.
Patients with genotype 1a, and CrCl < 30 mL/min for whom the urgency to treat is high and kidney transplant is not an immediate option, 12 weeks of daily co-formulated paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) and twice-daily dosed dasabuvir (250 mg) with RBV at reduced doses if hemoglobin > 10 g/dl (200 mg thrice weekly to daily) is recommended. (grade B2)
-
94.
Patients with genotype 4, and CrCl < 30 mL/min for whom the urgency to treat is high and kidney transplant is not an immediate option, 12 weeks of daily co-formulated paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) with RBV at reduced doses if hemoglobin > 10 g/dl (200 mg thrice weekly to daily) is recommended. (grade C2)
-
95.
Patients with HCV genotype 2, 3, 5, or 6 and CrCl below 30 mL/min for whom the urgency to treat is high and kidney transplant is not an immediate option, peginterferon and dose-adjusted RBV at 200 mg daily is recommended. (grade C2)
-
96.
Moderate renal impairment (with CrCl 30–50 mL/min), initial RBV dosing should be 200 mg or 400 mg every other day. (grade B2)
-
97.
For patients with severe renal impairment (with CrCl < 30 mL/min), or who are on hemodialysis initial RBV dosing should be 200 mg daily. (grade B2).
Treatment of patients with Cryoglobulinemia-associated glomerulonephritis
Cryoglobulinaemia refers to the presence of abnormal immunoglobulins in the serum, which have the unusual property of precipitating at temperatures below 37°C and dissolving again at higher temperatures. Cryoglobulins (CGs) are classified on the basis of their clonality into three types. Type II CGs and type III CGs (mixed cryoglobulinaemia (MC) ~29–54%) are highly prevalent in patients with chronic HCV infection.[168] In MC associated with systemic vasculitis induced by HCV infection through immune complexes depositions intravascularly and multiorgan involvement, treatment is based on treating the underlying etiology. Multiple treatment modalities have been used including antivirals, steroid therapy, and cyclophosphamide or plasma exchange.[169] Evidence is available for rituximab, however, its safety is questionable in the presence of new DAAs, however, it may improve outcome if it is proven to be effective. HCV infection and non-Hodgkin lymphoma (diffuse large B cell) have a significant association, and although rituximab treatment is favorable, it may cause viral replication as well as carry a risk of hepatotoxicity.
Recommendations
-
98.
Using new DAAs in treating HCV related lymphoma is warranted but the impact on SVR and on the prognosis is not clear. (grade C1)
-
99.
Treating mixed cryoglobulinemia and its associated renal disease requires more attention and further assessment when choosing a new DAA regimen and using rituximab in HCV-related renal disease. (grade C1).
Nonhepatic solid organ transplant recipients
Data on HCV following heart transplantation are scarce and controversial on improving survival. Risk of rejection using IFN-based therapy is unclear. Thus, treatment decisions should be based on IFN-free regimens and be decided on a case-by-case basis. HCV infection is considered a contraindication for lung transplantation.[170] No data on treating HCV after pancreases or small bowel transplantation could be found.
Recommendation
-
100.
Treatment of nonhepatic solid organ transplant recipients should be based on an interferon-free regimen and decided on a case-by-case basis with management of DDI with immunosuppressants. (grade C2).
Alcohol and people who inject drugs
Chronic alcohol consumption in patients with chronic hepatitis C is associated with an accelerated fibrosis progression, cirrhosis, and an increased risk of HCC.[171] SVR rates are lower in patients with alcohol abuse.[172] Illicit drug use by injection is the predominant mode of HCV transmission. The dual factors of being an active drug addict with chronic HCV infection carry a significant risk for advanced liver disease and liver- related mortality.[173] The prevalence of HCV among PWIDs is approximately 65%.[174]
Patients should be drug-free for at least 6 months before treatment and their treatment teams should be sure that they will adhere to treatment and regular follow-up visits. They also require close monitoring by an experienced multidisciplinary team.[175]
Provided there is a clear compliance to be treated to achieve SVR, treatment must be considered to reduce transmission.[176]
The new DAAs are associated with significantly less toxicity, which may improve treatment initiation and completion rate. It was shown in one study that OBV/PTV/r/DSV plus RBV regimen of 12 weeks achieved an SVR24 rate of 97.4% among GT1-infected patients receiving opioid replacement therapy. No viral breakthroughs or relapses were observed. The OBV/PTV/r/DSV plus RBV regimen was well-tolerated with low rates of discontinuation, and DDI were not found to impact HCV treatment or opioid maintenance.[177]
Data on DDI showed no significant interactions between SOF and SMV with methadone[178] and buprenorphine.[179] Midazolam and triazolam blood concentrations may increase with the use of SMV, and thus caution is warranted. No available or little data for DCV has been found to exist.
Recommendations
-
101.
Alcohol consumption should be strongly discouraged. (grade A1)
-
102.
The treatment should be individualized. (grade B1)
-
103.
Routine screening testing for HCV is warranted every 6–12 months in alcoholics and people who inject drugs. (grade B1)
-
104.
Pretreatment education about the disease and risk of transmission is crucial. (grade B1)
-
105.
If using opioid substitution therapy, patients should receive an interferon-free regimen. (grade B1)
-
106.
No dose adjustment is required for methadone and buprenorphine but monitoring of opioids toxicity or withdrawal is needed. More data is needed for daclatasvir. (grade B1)
-
107.
Opioid substitution therapy is not a contraindication for liver transplantation. (grade B1).
Treatment of patients with psychiatric illnesses
The use of IFN-based regimens has resulted in increasing concern regarding the psychiatric side effects that can result from this treatment. Significant depressive symptoms occur in 21–58% of patients.[180]
Former or active drug abuse and mental disorders are both considered risk factors. In addition, reports of suicide attempts during IFN therapy and the risk of reinfection has led to the opinion that the use of IFN is contraindicated for patients with a preexisting mental disorder, ongoing opiate abuse, or methadone substitution.
Recommendations
-
108.
Patients with HCV infection and concomitant mental and psychiatric disorders can be considered for treatment using the currently approved regimens. (grade C1)
-
109.
Treatment of hepatitis C infection in patients with psychiatric disorders should be undertaken only with the support of a multi-disciplinary team that should include psychiatric counseling services prior to therapy. (grade C1).
Hemoglobinopathies
The most frequent hemoglobinopathy associated with chronic hepatitis C is prevalent in countries where blood supply screening may be less stringent than in industrialized areas. As a result of repeated blood transfusions, thalassemia major is frequently associated with HCV infection as well as sickle cell anemia. The treatment of HCV in sickle cell patients poses a challenge to clinicians. An IFN-based regimen and RBV are usually avoided in these patients due to anemia. However, despite the absence of published data on the safety of an IFN and RBV-free regimen in these patients, it is not unreasonable to consider them for such treatment, especially because they do not aggravate anemia.
Recommendations
-
110.
Hemoglobinopathies are treated for the same indications. (grade A1)
-
111.
Although treatment regimens are the same as for normal persons, interferon-free regimen without vRBV can be considered. (grade B1)
-
112.
If RBV is to be used, blood transfusion might be needed. (grade B2)
-
113.
Careful monitoring for hematologic side effects is recommended. (grade C1).
Bleeding disorders
Hemophilia is an inherited bleeding disorder caused by a deficiency of either factor VIII or IX in hemophilia A and B, respectively. Treatment is based on intravenous replacement of these factors, which, until recently, were prepared from plasma donations. Hemophiliacs exposed to nonvirally inactivated concentrates prior to 1985 had an almost 100% chance of being infected with HCV with theirfirst exposure to concentrate. Other inherited bleeding disorders are treated with concentrates, including von Willebrand's disease and deficiencies of fibrinogen and factors II, VII, X, XI, and XIII.
Progression to end-stage liver disease is similar to that in HCV-positive individuals in the general population. The management of chronic hepatitis C in hemophilia is similar to that of the nonhemophilic population. New HCV DAAs are applicable to patients with hemophilia. Liver transplantation can provide a cure as a result of factor VIII production by the transplanted liver.
Recommendations
-
114.
The indications for HCV therapy are the same as in patients with and without bleeding disorders. (grade A1).
Acute hepatitis C
Infection lasting up to 6 months is considered acute, with spontaneous resolution occurring in 15–50%.[181] Most patients are asymptomatic, however, the expected rate of chronicity is 50–90%. The decision of when to treat such a patient is largely based on the likelihood of spontaneous resolution, and mainly to prevent progression. Spontaneous viral clearance has been associated with certain factors such as symptomatic disease, younger age, female gender, and IL28B gene polymorphism.
High SVR rates (>90%) have been reported with PegIFN monotherapy, regardless of the genotype, whereas lower rates with coinfected patients with HIV have been reported. Use of RBV showed no difference in SVR except in case of the presence of negative predictors such as HIV coinfection.[182]
Adding telaprevir in GT1 showed higher SVR rates.[183] There is as yet no clear strategy on when to start treatment but it is suggested to treat with ALT elevation.[184]
On the other hand, the fact that the majority of spontaneous resolution occurs within the first 12–16 weeks, treatment has been recommended within that window.[185] Others have suggested 4 weekly HCV RNA quantification and only to treat if the patient is still positive at 12 weeks.[186] There is no data to suggest treatment as post exposure prophylaxis. A few studies on the treatment of acute hepatitis C with DAA drugs are available. These studies confirmed the potency of the new medications in the eradication of HCV in its acute stage. For example, in a randomized open label prospective clinical pilot study, 29 patients with a diagnosis of acute hepatitis C received treatment. The patients were divided into 2 groups, group A (14 patients), who received a combination of LDV/SOF for 4 weeks and group B (15 patients), who received a combination of SOF and SMV for 8 weeks. SVR12 were 100% in both groups, with minimal adverse events. Further studies with larger number of patients are needed.[187]
Recommendations
-
115.
In acute HCV, observation of HCV RNA every 4–8 weeks for at least 12–16 weeks to detect spontaneous resolution before initiating treatment is recommended. (grade B1)
-
116.
When the decision is made to initiate treatment after 6 months, treatment as described for chronic hepatitis C is recommended. (grade C2)
-
117.
New DAAs used for chronic HCV cases can be used in acute HCV because they are expected to achieve high SVR rates. (grade B1)
-
118.
There is no treatment recommendation for post exposure prophylaxis. (grade B1).
CONCLUSIONS
The SASLT Guidelines for HCV provide a concise, updated, and evidence-based review of the diagnosis and management of chronic HCV infection in Saudi Arabia. This may help to initiate plans to screen and prevent HCV infection in the population, to bring about early and accurate diagnosis of patients with HCV infection, and to facilitate appropriate and timely referrals between primary, secondary, and tertiary care providers. With the increased efficacy of the new DAA treatments, which has been shown to be higher than 95%, liver-related mortality would be reduced and the goal of eradication of HCV will be achieved.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Abdo AA, Sanai FM, Al-Faleh FZ. Epidemiology of viral hepatitis in Saudi Arabia: Are we off the hook? Saudi J Gastroenterol. 2012;18:349–57. doi: 10.4103/1319-3767.103425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alswaidi FM, O’Brien SJ. Is there a need to include HIV, HBV and HCV viruses in the Saudi premarital screening program on the basis of their prevalence and transmission risk factors? J Epidemiol Community Health. 2010;64:989–97. doi: 10.1136/jech.2009.093302. [DOI] [PubMed] [Google Scholar]
- 3.Al Traif I, Al Balwi MA, Abdulkarim I, Handoo FA, Alqhamdi HS, Alotaibi M, et al. HCV genotypes among 1013 Saudi nationals: A multicenter study. Ann Saudi Med. 2013;33:10–2. doi: 10.5144/0256-4947.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Ashgar HI, Khan MQ, Al-Ahdal M, Al Thawadi S, Helmy AS, Al Qahtani A, et al. Hepatitis C genotype 4: Genotypic diversity, epidemiological profile, and clinical relevance of subtypes in Saudi Arabia. Saudi J Gastroenterol. 2013;19:28–33. doi: 10.4103/1319-3767.105920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanai FM, Helmy A, Dale C, Al-Ashgar H, Abdo AA, Katada K, et al. Updated thresholds for alanine aminotransferase do not exclude significant histological disease in chronic hepatitis C. Liver Int. 2011;31:1039–46. doi: 10.1111/j.1478-3231.2011.02551.x. [DOI] [PubMed] [Google Scholar]
- 6.Colin C, Lanoir D, Touzet S, Meyaud-Kraemer L, Bailly F, Trepo C. Sensitivity and specificity of third-generation hepatitis C virus antibody detection assays: An analysis of the literature. J Viral Hepat. 2001;8:87–95. doi: 10.1046/j.1365-2893.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 7.Ramia S, Eid-Fares J. Distribution of hepatitis C virus genotypes in the Middle East. Int J Infect Dis. 2006;10:272–7. doi: 10.1016/j.ijid.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Shiffman ML, Diago M, Tran A, Pockros P, Reindollar R, Prati D, et al. Chronic hepatitis C in patients with persistently normal alanine transaminase levels. Clin Gastroenterol Hepatol. 2006;4:645–52. doi: 10.1016/j.cgh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Chevaliez S, Pawlotsky JM. Diagnosis and management of chronic viral hepatitis: Antigens, antibodies and viral genomes. Best Pract Res Clin Gastroenterol. 2008;22:1031–48. doi: 10.1016/j.bpg.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Afdhal NH, Nunes D. Evaluation of liver fibrosis: A concise review. Am J Gastroenterol. 2004;99:1160–74. doi: 10.1111/j.1572-0241.2004.30110.x. [DOI] [PubMed] [Google Scholar]
- 11.Poynard T, Imbert-Bismut F, Munteanu M, Messous D, Myers RP, Thabut D, et al. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV Fibrosure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol. 2004;3:8. doi: 10.1186/1476-5926-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Ghamdi AS. Fibroscan®: A noninvasive test of liver fibrosis assessment. Saudi J Gastroenterol. 2007;13:147–9. doi: 10.4103/1319-3767.33470. [DOI] [PubMed] [Google Scholar]
- 13.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–93. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 14.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, et al. Non-invasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 15.Simmonds P, Alberti A, Alter HJ, Bonino F, Bradley DW, Brechot C et al. A proposed system for nomenclature of hepatitis C viral genotypes. Hepatology. 1994;19:1321–4. [PubMed] [Google Scholar]
- 16.Moradpour D, Penin F. Hepatitis C virus proteins: From structure to function. Curr Top Microbiol Immunol. 2013;369:113–42. doi: 10.1007/978-3-642-27340-7_5. [DOI] [PubMed] [Google Scholar]
- 17.Lohmann V. Hepatitis C virus RNA replication. Curr Top Microbiol Immunol. 2013;369:167–98. doi: 10.1007/978-3-642-27340-7_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choo, QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–62. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 19.Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–3. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 20.Bartosch B, Dubuisson J, Cosset FL. Infectious hepatitis C pseudo-particles containing functional E1E2 envelope protein complexes. J Exp Med. 2003;197:633–42. doi: 10.1084/jem.20021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindenbach BD, Evans MJ, Syder AJ, Wölk B, Tellinghuisen TL, Liu CC, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–6. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 22.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nature Med. 2005;11:791–6. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. New Engl J Med. 1998;339:1485–92. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 24.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa- 2b plus ribavirin for initial treatment of chronic hepatitis C: A randomized trial. Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. New Engl J Med. 2011;364:2405–16. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 26.Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. New Engl J Med. 2011;364:1195–206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinot-Peignoux M, Stern C, Mayli S, Ripault MP, Boyer N, Leclere L, et al. Twelve weeks post treatment follow-up is as relevant as 24 weeks to determine the sustained virologic response in patients with hepatitis C virus receiving pegylated interferon and ribavirin. Hepatology. 2010;51:1122–6. doi: 10.1002/hep.23444. [DOI] [PubMed] [Google Scholar]
- 28.Swain MG, Lai MY, Shiffman ML, Cooksley WG, Zeuzem S, Dieterich DT, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593–601. doi: 10.1053/j.gastro.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: A cure and so much more. Clin Infect Dis. 2011;52:889–900. doi: 10.1093/cid/cir076. [DOI] [PubMed] [Google Scholar]
- 30.Maylin S, Martinot-Peignoux M, Moucari R, Boyer N, Ripault MP, Cazals-Hatem D, et al. Eradication of hepatitis C virus in patients successfully treated for chronic hepatitis C. Gastroenterology. 2008;135:821–9. doi: 10.1053/j.gastro.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 31.Moon C, Jung KS, Kim do Y, Baatarkhuu O, Park JY, Kim BK, et al. Lower incidence of hepatocellular carcinoma and cirrhosis in hepatitis C patients with sustained virological response by pegylated interferon and ribavirin. Dig Dis Sci. 2014;60:573–81. doi: 10.1007/s10620-014-3361-6. [DOI] [PubMed] [Google Scholar]
- 32.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. J Am Med Assoc. 2012;308:2584–93. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 33.You D, Pockros P. Simeprevir for the treatment of chronic hepatitis C. Expert Opin Pharmacother. 2013;14:2581. doi: 10.1517/14656566.2013.850074. [DOI] [PubMed] [Google Scholar]
- 34.Gentile I, Borgia F, Buonomo A, Zappulo E, Castaldo G, Borgia G. ABT-450: A novel protease inhibitor for the treatment of hepatitis C virus infection. Curr Med Chem. 2014;21:3261. doi: 10.2174/0929867321666140706125950. [DOI] [PubMed] [Google Scholar]
- 35.Sofia M. Beyond sofosbuvir: What opportunity exists for a better nucleoside/nucleotide to treat hepatitis C? Antivir Res. 2014;107:119. doi: 10.1016/j.antiviral.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Gentile I, Buonomo A, Borgia G. Dasabuvir: A non-nucleoside inhibitor of NS5B for the treatment of hepatitis C virus infection. Rev Recent Clin Trials. 2014;9:115–23. doi: 10.2174/1574887109666140529222602. [DOI] [PubMed] [Google Scholar]
- 37.Aghemo A, De Francesco R. Daclatasvir: A team player rather than a prima donna in the treatment of hepatitis C. Gut. 2015;64:860–2. doi: 10.1136/gutjnl-2014-307958. [DOI] [PubMed] [Google Scholar]
- 38.Gentile I, Buonomo A, Borgia F, Castaldo G, Borgia G. Ledipasvir: A novel synthetic antiviral for the treatment of HCV infection. Expert Opin Investig Drugs. 2014;23:561. doi: 10.1517/13543784.2014.892581. [DOI] [PubMed] [Google Scholar]
- 39.Stirnimann G. Ombitasvir (ABT-267), a novel NS5A inhibitor for the treatment of hepatitis C. Expert Opin Pharmacother. 2014;15:2609–22. doi: 10.1517/14656566.2014.972364. [DOI] [PubMed] [Google Scholar]
- 40.Kenilworth, NJ, USA: Merck Sharp and Dohme Corp; 2016. Merck & Co., Inc. Elbasvir and Grazoprevir [package insert] [Google Scholar]
- 41.Sidharthan S, Kohli A, Sims Z, Nelson A, Osinusi A, Masur H, et al. Utility of hepatitis C viral load monitoring on direct-acting antiviral therapy. Clin Infect Dis. 2015;60:1743. doi: 10.1093/cid/civ170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrington PR, Deming DJ, Komatsu TE, Naeger LK. Hepatitis C virus RNA levels during interferon-free combination direct-acting antiviral treatment in registrational trials. Clin Infect Dis. 2015;61:666–7. doi: 10.1093/cid/civ402. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida EM, Sulkowski MS, Gane EJ, Herring RW Jr, Ratziu V, Ding X, et al. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology. 2015;61:41. doi: 10.1002/hep.27366. [DOI] [PubMed] [Google Scholar]
- 44.Recommendations for Testing, Managing, and Treating Hepatitis C. Joint panel from the American Association of the Study of Liver Diseases and the Infectious Diseases Society of America. 2014. Jan, [Last accessed on 2015 Jan 01]. Available from http://www.hcvguidelines.org/
- 45.European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63:199. doi: 10.1016/j.jhep.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 46.Miller MH, Agarwal K, Austin A, Brown A, Barclay ST, Dundas P, et al. Review article: 2014 UK consensus guidelines-Hepatitis C management and direct-acting anti-viral therapy. Aliment Pharmacol Ther. 2014;39:1363. doi: 10.1111/apt.12764. [DOI] [PubMed] [Google Scholar]
- 47.Chou R, Hartung D, Rahman B, Wasson N, Cottrell EB, Fu R. Comparative effectiveness of antiviral treatment for hepatitis C virus infection in adults: A systematic review. Ann Intern Med. 2013;158:114. doi: 10.7326/0003-4819-158-2-201301150-00576. [DOI] [PubMed] [Google Scholar]
- 48.Jacobson IM, Asante-Appiah E, Wong P, Black TA, Howe AY, Wahl J, et al. San Francisco, CA: 2015b. Prevalence and Impact of Baseline NSA Resistance Associated Variants (RAVs) on the Efficacy of Elbasvir/Grazoprevir (EBR/GZR) Against GT1a Infection [Abstract LB-22]. 66th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD). November 13-17. [Google Scholar]
- 49.Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: The COSMOS randomised study. Lancet. 2014;384:1756. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 50.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 51.Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 52.Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–93. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 53.Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): An open-label, randomised, phase 2 trial. Lancet. 2014;383:515–23. doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 54.Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Subramanian GM, et al. Efficacy of nucleotide polymerase inhibitor sofosbuvir plus the NS5A inhibitor ledipasvir or the NS5B non-nucleoside inhibitor GS-9669 against HCV genotype 1 infection. Gastroenterology. 2014;146:736–43. doi: 10.1053/j.gastro.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 55.Bourlière M, Bronowicki JP, de Ledinghen V, Hézode C, Zoulim F, Mathurin P, et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: A randomised, double-blind, phase 2 trial (SIRIUS) Lancet Infect Dis. 2015;15:397–404. doi: 10.1016/S1473-3099(15)70050-2. [DOI] [PubMed] [Google Scholar]
- 56.Reddy KR, Bourlière M, Sulkowski M, Omata M, Zeuzem S, Feld JJ, et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: An integrated safety and efficacy analysis. Hepatology. 2015;62:79–86. doi: 10.1002/hep.27826. [DOI] [PubMed] [Google Scholar]
- 57.Osinusi A, Kohli A, Marti MM, Nelson A, Zhang X, Meissner EG, et al. Re-treatment of chronic hepatitis C virus genotype 1 infection after relapse: An open-label pilot study. Ann Intern Med. 2014;161:634–8. doi: 10.7326/M14-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wyles D, Pockros P, Morelli G, Younes Z, Svarovskaia E, Yang JC, et al. Ledipasvir-sofosbuvir plus ribavirin for patients with genotype 1 hepatitis C virus previously treated in clinical trials of sofosbuvir regimens. Hepatology. 2015;61:1793–7. doi: 10.1002/hep.27814. [DOI] [PubMed] [Google Scholar]
- 59.Hézode C, Fontaine H, Dorival C, Larrey D, Zoulim F, Canva V, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC)- NCT01514890. J Hepatol. 2013;59:434–41. doi: 10.1016/j.jhep.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 60.Mizokami M, Yokosuka O, Takehara T, Sakamoto N, Korenaga M, Mochizuki H, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: An open-label, randomised, phase 3 trial. Lancet Infect Dis. 2015;15:645–53. doi: 10.1016/S1473-3099(15)70099-X. [DOI] [PubMed] [Google Scholar]
- 61.Bourliere M, Sulkowski M, Omata M, Zeuzem S, Feld J, Lawitz E, et al. An integrated safety and efficacy analysis of >500 patients with compensated cirrhosis treated with ledipasvir/sofosbuvir with or without ribavirin. Presented at the American Association for the Study of Liver Diseases Liver Meeting, Boston MA, November 7–11. 2014 Abstract #82. [Google Scholar]
- 62.Wyles DL, Pockros PJ, Yang JC, Zhu Y, Pang PS, McHutchison JG, et al. Retreatment of patients who failed prior sofosbuvir-based regimens with all oral fixed-dose combination ledipasvir-sofosbuvir plus ribavirin for 12 weeks. Hepatology. 2014;60:317A. [Google Scholar]
- 63.Feld JJ, Moreno C, Trinh R, Tam E, Bourgeois S, Horsmans Y, et al. Turquoise-III: Safety and efficacy of 12-week ribavirin-free treatment for patients with HCV genotype 1b and cirrhosis. Presented at the 15th Annual International Symposium on Viral Hepatitis and Liver Disease. Berlin, Germany June 26-28. 2015 Abstract #P226. [Google Scholar]
- 64.Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson D, Crawford D, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 65.Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973–82. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 66.Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–92. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 67.Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourlière M, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1604–14. doi: 10.1056/NEJMoa1401561. [DOI] [PubMed] [Google Scholar]
- 68.Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, Maieron A, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147:359–65. doi: 10.1053/j.gastro.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 69.Lawitz E, Makara M, Akarca US, Thuluvath PJ, Preotescu LL, Varunok P, et al. Efficacy and Safety of Ombitasvir, Paritaprevir, and Ritonavir in an Open-Label Study of Patients with Genotype 1b Chronic Hepatitis C Virus Infection with and Without Cirrhosis. Gastroenterology. 2015;149:971–80. doi: 10.1053/j.gastro.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Lawitz E, Hezode C, Varunok P, Thuluvath PJ, Baykal T, Kapoor M, et al. Interferon- and ribavirin-free regimen of ABT-450/r+ABT-267 in HCV genotype 1b infected treatment naive patients and prior null responders. Presented at the 64th annual meeting of the American Association for the Study of Liver Diseases, Washington, DC, November 1–5. 2013 [Google Scholar]
- 71. [Accessed on January 01, 2015]. VIEKIRA PAK (ombitasvir, paritaprevir, and ritonavir tablets; dasabuvir tablets). US FDA approved product information. National Library of Medicine. Available from www.dailymed.nlm.nih.gov . [Google Scholar]
- 72.Zeuzem S, Ghalib R, Reddy KR, Pockros PJ, Ari ZB, Zhao Y, et al. Grazoprevir-Elbasvir Combination Therapy for Treatment-Naive Cirrhotic and Noncirrhotic Patients with Chronic Hepatitis C Virus Genotype 1, 4, or 6 Infection: A Randomized Trial. Ann Intern Med. 2015;163:1–13. doi: 10.7326/M15-0785. [DOI] [PubMed] [Google Scholar]
- 73.Sulkowski M, Hezode C, Gerstoft J, Vierling JM, Mallolas J, Pol S, et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): A randomised, open-label phase 2 trial. Lancet. 2015b;385:1087–97. doi: 10.1016/S0140-6736(14)61793-1. [DOI] [PubMed] [Google Scholar]
- 74.Pearlman BL, Ehleben C, Perrys M. The combination of simeprevir and sofosbuvir is more effective than that of peginterferon, ribavirin, and sofosbuvir for patients with hepatitis C-related Child's class A cirrhosis. Gastroenterology. 2015;148:762–70. doi: 10.1053/j.gastro.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 75.Kwo P, Gitlin N, Nahass R, Bernstein D, Rojter S, Schiff E, et al. A phase 3, randomised, open-label study to evaluate the efficacy and safety of 8 and 12 weeks of simeprevir (SMV) plus sofosbuvir (SOF) in treatment-naive and -experienced patients with chronic HCV genotype 1 infection without cirrhosis: OPTIMIST-1. J Hepatol. 2015;62:S270. [Google Scholar]
- 76.Lawitz E, Matusow G, DeJesus E, Yoshida E, Felizarta F, Ghalib R, et al. A phase 3, open-label, single-arm study to evaluate the efficacy and safety of 12 weeks of simeprevir (SMV) plus sofosbuvir (SOF) in treatment-naive or -experienced patients with chronic HCV genotype 1 infection and cirrhosis: OPTIMIST-2. Presented at the 50th Annual Meeting of the European Association for the Study of the Liver (EASL), Vienna Austria, April 22–26. 2015 [Google Scholar]
- 77.Jesnsen DM, O’Leary J, Pockros, P, Sherman K, Kwo P, Mailliard M, et al. Safety and efficacy of sofosbuvir-containing regimens for hepatitis C: Real world experience in a diverse, longitudinal observational cohort. Presented at the American Association for the Study of Liver Diseases Liver Meeting, Boston MA, November 7–11. 2014 [Google Scholar]
- 78.Dieterich D, Bacon BR, Flamm SL, Kowdley KV, Milligan S, Tsai N, et al. Evaluation of sofosbuvir and simeprevir-based regimens in the TRIO network: Academic and community treatment of a real-world, hetero-geneous population. Hepatology. 2014;60:220A. [Google Scholar]
- 79.Aqel BA, Pungpapong S, Leise M, Werner KT, Chervenak AE, Watt KD, et al. Multicenter experience using simeprevir and sofosbuvir with or without ribavirin to treat hepatitis C genotype 1 in patients with cirrhosis. Hepatology. 2015;62:1004–12. doi: 10.1002/hep.27937. [DOI] [PubMed] [Google Scholar]
- 80.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211–21. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 81.Wyles DL, Ruane PJ, Sulkowski MS, Luetkemeyer A, Morgan TR, Sherman KE, et al. Daclatasvir plus Sofosbuvir for HCV in Patients Coinfected with HIV-1. N Engl J Med. 2015;373:714–25. doi: 10.1056/NEJMoa1503153. [DOI] [PubMed] [Google Scholar]
- 82.Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, et al. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology. 2016;63:1493–505. doi: 10.1002/hep.28446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lawitz E, Lalezari JP, Hassanein T, Kowdley KV, Poordad FF, Sheikh AM, et al. Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: A randomised, double-blind, phase 2 trial. Lancet Infect Dis. 2013;13:401–8. doi: 10.1016/S1473-3099(13)70033-1. [DOI] [PubMed] [Google Scholar]
- 84.Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, DeMicco M, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): An open-label, randomised, multicentre phase 2 trial. Lancet. 2013;381:2100–7. doi: 10.1016/S0140-6736(13)60247-0. [DOI] [PubMed] [Google Scholar]
- 85.Hayashi N, Izumi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, et al. Simeprevir with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1 patients in Japan: CONCERTO-1, a phase III trial. J Hepatol. 2014;61:219–27. doi: 10.1016/j.jhep.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 86.Forns X, Lawitz E, Zeuzem S, Gane E, Bronowicki JP, Andreone P, et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: A phase 3 trial. Gastroenterology. 2014;146:1669–79. doi: 10.1053/j.gastro.2014.02.051. [DOI] [PubMed] [Google Scholar]
- 87.Zeuzem S, Berg T, Gane E, Ferenci P, Foster GR, Fried MW, et al. Simeprevir increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: A phase IIb trial. Gastroenterology. 2014;146:430–41. doi: 10.1053/j.gastro.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 88.Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403–13. doi: 10.1016/S0140-6736(14)60494-3. [DOI] [PubMed] [Google Scholar]
- 89.Manns M, Marcellin P, Poordad F, de Araujo ES, Buti M, Horsmans Y, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414–26. doi: 10.1016/S0140-6736(14)60538-9. [DOI] [PubMed] [Google Scholar]
- 90.Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77–87. doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–87. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 92.Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993–2001. doi: 10.1056/NEJMoa1316145. [DOI] [PubMed] [Google Scholar]
- 93.Esteban R, Nyberg L, Lalezari J, Ni L, Doehle B, Kanwar B, et al. Successful retreatment with sofosbuvir-containing regimens for HCV genotype 2 or 3 infected patients who failed prior sofosbuvir plus ribavirin therapy. JHepatol. 2014;60:S4. [Google Scholar]
- 94.Lawitz E, Poordad F, Brainard DM, Hyland RH, An D, Symonds WT, et al. Sofosbuvir in combination with PegIFN and ribavirin for 12 weeks provides high SVR rates in HCV-infected genotype 2 or 3 treatment-experienced patients with and without compensated cirrhosis: Results from the LONESTAR-2 study. Hepatology. 2013;58:1380A. [Google Scholar]
- 95.Foster GR, Pianko S, Brown A, Forton D, Nahass RG, George J, et al. Efficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in patients with HCV genotype 3 infection and treatment-experienced patients with cirrhosis and HCV genotype 2 infection. Gastroenterology. 2015;149:1462–70. doi: 10.1053/j.gastro.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 96.Brown A, Hezode C, Zuckerman E, Foster G, Zekry A, Roberts S, Howe A, et al. C-SCAPE: Efficacy and safety of 12 weeks of grazoprevir+elbasvir+ribavirin in patients with HCV GT2, 4, or 6 infection. Presented at the European Association for the Study of the Liver (EASL) 50th International Liver Congress 2015. Vienna, Austria April 22–26. 2015 Poster P0771. [Google Scholar]
- 97.Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127–35. doi: 10.1002/hep.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leroy V, Angus P, Bronowicki JP, Dore GJ, Hezode C, Pianko S, et al. Daclatasvir, Sofosbuvir, and Ribavirin for Hepatitis C Virus Genotype 3 and Advanced Liver Disease: A Randomized Phase III Study (ALLY-3+) Hepatology. 2016;63:1430–41. doi: 10.1002/hep.28473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, et al. Daclatasvir with Sofosbuvir and Ribavirin for HCV Infection with Advanced Cirrhosis or Post-Liver Transplant Recurrence. Hepatology. 2016;63:1493–505. doi: 10.1002/hep.28446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wong KA, Worth A, Martin R, Svarovskaia E, Brainard DM, Lawitz E, et al. Characterization of Hepatitis C virus resistance from a multiple-dose clinical trial of the novel NS5A inhibitor GS-5885. Antimicrob Agents Chemother. 2013;57:6333–40. doi: 10.1128/AAC.02193-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kamal SM, Nasser IA. Hepatitis C genotype 4: What we know and what we don’t yet know. Hepatology. 2008;47:1371–83. doi: 10.1002/hep.22127. [DOI] [PubMed] [Google Scholar]
- 102.Abdel-Ghaffar TY, Sira MM, El Naghi S. Hepatitis C genotype 4: The past, present, and future. World J Hepatol. 2015;7:2792–810. doi: 10.4254/wjh.v7.i28.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aghemo A, Rumi MG, Monico S, Prati GM, D’Ambrosio R, Donato MF, et al. The pattern of pegylated interferon-alpha2b and ribavirin treatment failure in cirrhotic patients depends on hepatitis C virus genotype. Antivir Ther. 2009;14:577–84. [PubMed] [Google Scholar]
- 104.Hézode C, Asselah T, Reddy KR, Hassanein T, Berenguer M, Fleischer-Stepniewska K, et al. Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL-I): A randomized, open-label trial. Lancet. 2015;385:2502–9. doi: 10.1016/S0140-6736(15)60159-3. [DOI] [PubMed] [Google Scholar]
- 105.Asselah T, Hassanien TI, Qaqish RB, Feld JJ, Hezode C, Zeuzem S, et al. San Francisco, CA: 2015a. Efficacy and Safety of Ombitasvir/Paritaprevir/Ritonavir Co-Administered with Ribavirin in Adults with Genotype 4 Chronic Hepatitis C Infection and Cirrhosis (AGATE-I) [Abstract 714]. 66th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD). November 13–17. [Google Scholar]
- 106.Esmat G, Doss W, Qqish RB, Waked I, Shiha GE, Yosry A, et al. San Francisco, CA: 2015. Efficacy and Safety of Co-Formulated Ombitasvir/Paritaprevir/Ritonavir with Ribavirin in Adults with Chronic HCV Genotype 4 Infection in Egypt (AGATE-II) [Abstract 708]. 66th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD). November 13–17. [Google Scholar]
- 107.Zeuzem S, Ghalib R, Reddy KR, Pockros PJ, Ben Ari Z, Zhao Y, et al. Combination Therapy for Treatment-Naive Cirrhotic and Noncirrhotic Patients With Chronic Hepatitis C Virus Genotype 1, 4, or 6 Infection: A Randomized Trial. Ann Intern Med. 2015;163:1–13. doi: 10.7326/M15-0785. [DOI] [PubMed] [Google Scholar]
- 108.Asselah T, Reesink H, Gerstoft J, de Ledinghen V, Pockros P, Robertson M, et al. San Francisco, CA: 2015. High Efficacy of Elbasvir and Grazoprevir with or without Ribavirin in 103 Treatment-Naïve and Experienced Patients with HCV Genotype 4 infection: A Pooled Analysis. [Abstract 251]. 66th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD) November 13–17. [Google Scholar]
- 109.Kohli A, Kapoor R, Sims Z, Nelson A, Sidharthan S, Lam B, et al. Ledipasvir and sofosbuvir for hepatitis C genotype 4: A proof-of-concept, single-centre, open-label phase 2a cohort study. Lancet Infect Dis. 2015;15:1049–54. doi: 10.1016/S1473-3099(15)00157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith MA, Mohammad RA. Ledipasvir-sofosbuvir for hepatitis C genotype 4 infection. Lancet Infect Dis. 2015;15:993–5. doi: 10.1016/S1473-3099(15)00223-6. [DOI] [PubMed] [Google Scholar]
- 111.Abergel A, Loustaud-Ratti V, Metivier S, Jiang D, Kersey K, Knox SJ, et al. Vienna, Austria: 2015. Ledipasvir/sofosbuvir for the treatment of patients with chronic genotype 4 or 5 HCV infection. 50th Annual Meeting of the European Association for the Study of the Liver (EASL). April 22–26. [Google Scholar]
- 112.Naggie S, Cooper C, Saag M, Workowski K, Ruane P, Towner WJ, et al. Ledipasvir and Sofosbuvir for HCV in Patients Coinfected with HIV-1. N Engl J Med. 2015;373:705–13. doi: 10.1056/NEJMoa1501315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS, Jr, et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients with Advanced Liver Disease. Gastroenterology. 2015;149:649–59. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 114.Ruane PJ, Ain D, Stryker R, Meshrekey R, Soliman M, Wolfe PR, et al. Sofosbuvir plus ribavirin for the treatment of chronic genotype 4 hepatitis C virus infection in patientsof Egyptian ancestry. J Hepatol. 2015;62:1040–6. doi: 10.1016/j.jhep.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 115.Doss W, Shiha G, Hassany M, Soliman R, Fouad R, Samir W, et al. Sofosbuvir plus ribavirin for treating Egyptian patients with hepatitis C genotype 4. J Hepatol. 2015;63:581–5. doi: 10.1016/j.jhep.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 116.Molina JM, Orkin C, Iser DM, Zamora FX, Nelson M, Stephan C, et al. Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): A multicentre, open-label, non-randomised, phase 3 study. Lancet. 2015;385:1098–106. doi: 10.1016/S0140-6736(14)62483-1. [DOI] [PubMed] [Google Scholar]
- 117.Welzel TM, Herzer K, Ferenci P, Petersen J, Gschwantler M, Cornberg M, et al. Vienna, Austria: 2015. Daclatasvir plus sofosbuvir with or without ribavirin for the treatment of HCV in patients with severe liver disease: Interim results of a multicenter compassionate use programe-Poster presented at, The International Liver Congress™ 2015, 50th annual meeting of the European Association for the Study of the Liver (EASL); April 22–26. Poster P0772. [Google Scholar]
- 118.Hézode C, Abergel A, Chas J, Conti F, Cotte L, Tateo M, et al. Sustained virologic response to Daclatasvir and Sofosbuvir with or without Ribavarin, among patients in the French Daclatasvir ATU programme infected with HCV genotypes 4, 5 and 6. EASL meeting 2016 Poster SAT-131 [Google Scholar]
- 119.El Raziky M, Gamil M, Hammad R, Hashem M, Marwa Khairy M. Treatment of Hepatitis C Genotype 4 patients with Simeprevir and Sofosbuvir: Preliminary Results from a Phase IIa, Partially Randomised, Open-label Trial conducted in Egypt (OSIRIS), AASLD 2015 Abstract # 1163 [Google Scholar]
- 120.Moreno C, Lasser L, Delwaide J, Starkel P, Laleman W, Langlet P, et al. Sofosbuvir in combination with simeprevir +/- ribavirin in genotype 4 hepatitis C patients with advanced fibrosis or cirrhosis: Real-life experience from Belgium. Abstract#1091 AASLD 2015. doi: 10.1371/journal.pone.0170933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kayali Z, Amador C, Lowe A, Ashouri B, Lam K, Schmidt WN, et al. Prospective Study for The Efficacy of Sofosbuvir and Simeprevir±Ribavirin in Hepatitis C Genotype 1 and 4 Compensated Cirrhotic Patients. Single Center Study and Real Life Experience. AASLD. 2015 Abstract # 1100. [Google Scholar]
- 122.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–87. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 123.Osoba AO. Hepatitis C virus genotypes in Saudi Arabia. Saudi Med J. 2002;23:7–12. [PubMed] [Google Scholar]
- 124.Abergel A, Asselah T, Metivier S, Kersey K, Jiang D, Mo H, et al. Ledipasvir-sofosbuvir in patients with hepatitis C virus genotype 5 infection: An open-label, multicentre, single-arm, phase 2 study. Lancet Infect Dis. 2016;4:459–64. doi: 10.1016/S1473-3099(15)00529-0. [DOI] [PubMed] [Google Scholar]
- 125.Hu P, Chen Z, Li H, Ren H. San Francisco, California: 2015. Global prevalence of pre-existing hepatitis C virus resistant-associated variants to direct-acting antiviral agents (DAA): Mining of GenBank HCV genome data. Program and abstracts of the 66th Annual Meeting of the American Association for the Study of Liver Diseases; November 14–17. Abstract 1044. [Google Scholar]
- 126.Zeuzem S, Mizokami M, Pianko S, Mangia A, Han KH, Martin R, et al. San Francisco, California: 2015. Prevalence of pre-treatment NS5A resistance associated variants in genotype 1 patients across different regions using deep sequencing and effect on treatment outcome with LDV/SOF. Program and abstracts of the 66th Annual Meeting of the American Association for the Study of Liver Diseases; November 14–17. Abstract 91. [Google Scholar]
- 127.Dvory-Sobol H, Wyles D, Ouyang W, Chodavarapu K, McNally J, Cheng W, et al. Long-term persistence of HCV NS5A variants after treatment with NS5A inhibitor ledipasvir. J Hepatol. 2015;62:S221. [Google Scholar]
- 128.Sulkowski MS, Brau N, Lawitz E, Shiffman ML, Towner WJ, Ruane PJ, et al. San Francisco, California: 2015. A randomized controlled trial of sofosbuvir/GS-5816 fixed dose combination for 12 weeks compared to sofosbuvir with ribavirin for 12 weeks in genotype 2 HCV infected patients: The phase 3 ASTRAL-2 study. Program and abstracts of the 66th Annual Meeting of the American Association for the Study of Liver Diseases; November 14–17. Abstract 205. [Google Scholar]
- 129.Mangia A, Roberts SK, Pianko S, Thompson A, Cooper C, Conway B, et al. San Francisco, California: 2015. Sofosbuvir/GS-5816 fixed dose combination for 12 weeks compared to sofosbuvir with ribavirin for 24 weeks in genotype 3 HCV infected patients: The randomized controlled phase 3 ASTRAL-3 study. Program and abstracts of the 66th Annual Meeting of the American Association for the Study of Liver Diseases; November 14–17. Abstract 249. [Google Scholar]
- 130.AASLD/IDSA/IAS–USA. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Available from: http://www.hcvguidelines.org/full-report-view . March 2016 Version.
- 131.Barnard RJ, Howe JA, Ogert RA, Zeuzem S, Poordad F, Gordon SC, et al. Analysis of boceprevir resistance associated amino acid variants (RAVs) in two phase 3 boceprevir clinical studies. Virology. 2013;444:329–36. doi: 10.1016/j.virol.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 132.McPhee F, Hernandez D, Yu F, Ueland J, Monikowski A, Carifa A, et al. Resistance analysis of hepatitis C virus genotype 1 prior treatment null responders receiving daclatasvir and asunaprevir. Hepatology. 2013;58:902–11. doi: 10.1002/hep.26388. [DOI] [PubMed] [Google Scholar]
- 133.Sullivan JC, De Meyer S, Bartels DJ, Dierynck I, Zhang EZ, Spanks J, et al. Evolution of treatment-emergent resistant variants in telaprevir phase 3 clinical trials. Clin Infect Dis. 2013;57:221–9. doi: 10.1093/cid/cit226. [DOI] [PubMed] [Google Scholar]
- 134.Wang C, Sun JH, O’Boyle DR, 2nd, Nower P, Valera L, Roberts S, et al. Persistence of resistant variants in hepatitis C virus-infected patients treated with the NS5A replication complex inhibitor daclatasvir. Antimicrob Agents Chemother. 2013;57:2054–65. doi: 10.1128/AAC.02494-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lenz O, Verbinnen T, Fevery B, Tambuyzer L, Vijgen L, Peeters M, et al. Virology analyses of HCV isolates from genotype 1-infected patients treated with simeprevir plus peginterferon/ribavirin in Phase IIb/III studies. J Hepatol. 2015;62:1008–14. doi: 10.1016/j.jhep.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 136.Krishnan P, Tripathi R, Schnell G, Reisch T, Beyer J, Irvin M, et al. Pooled analysis of resistance in patients treated with ombitasvir/ABT-450/r and dasabuvir with or without ribavirin in Phase 2 and Phase 3 clinical trials. Hepatology. 2014;60:1134A. [Google Scholar]
- 137.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: A meta-analysis of observational studies. Ann Intern Med. 2013;158:329–37. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 138.Singal AK, Freeman DH, Jr, Anand BS. Meta-analysis: Interferon improves outcomes following ablation or resection of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;32:851–858. doi: 10.1111/j.1365-2036.2010.04414.x. [DOI] [PubMed] [Google Scholar]
- 139.Gane E, Pilmore H. Management of chronic viral hepatitis before and after renal transplantation. Transplantation. 2002;74:427–37. doi: 10.1097/00007890-200208270-00001. [DOI] [PubMed] [Google Scholar]
- 140.Flamm SL, Everson GT, Charlton M, Denning JM, Arterburn S, Brandt-Sarif T, et al. Ledipasvir/sofosbuvir with ribavirin for the treatment of HCV in patients with decompensated cirrhosis: Preliminary results of a prospective, multicenter study. Hepatology. 2014;60:320A. [Google Scholar]
- 141.Afdhal N, Everson G, Calleja JL, McCaughan G, Symonds WT, Denning J, et al. Sofosbuvir and ribavirin for the treatment of chronic HCV with cirrhosis and portal hypertension with and without decompensation: Early virologic response and safety. J Hepatol. 2014;60:S28. [Google Scholar]
- 142.Rockstroh J, Welzel TM, Ingiliz P, Petersen J, Van der Valk M, Herzer K, et al. Boston, MA: 2014. Daclatasvir Plus Sofosbuvir with or Without Ribavirin for the Treatment of Chronic HCV in Patients Coinfected with HIV: Interim Results of a Multicenter European Compassionate Use Program. [Abstract 1058] 65th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD). November 7–11. [Google Scholar]
- 143.Welzel TM, Petersen J, Ferenci P, Gschwantler M, Herzer K, Cornberg M, et al. Boston, MA: 2014. Safety and Efficacy of Daclatasvir Plus Sofosbuvir With or Without Ribavirin for the Treatment of Chronic HCV Genotype 3 Infection: Interim Results of a Multicenter European Compassionate Use Program. [Abstract] 65th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD). November 7–11. [Google Scholar]
- 144.Curry MP, Forns X, Chung RT, Terrault NA, Brown Jr R, Fenkel JM, et al. Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: An open-label study. Gastroenterology. 2015;148:100–7. doi: 10.1053/j.gastro.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 145.Jensen DM, O’Leary JG, Pockros PJ, Sherman KE, Kwo PY, Mailliard ME, et al. Safety and efficacy of sofosbuvir-containing regimens for hepatitis C: Real- world experience in a diverse, longitudinal observational cohort. Hepatology. 2014;60:219A. [Google Scholar]
- 146.Reddy R, Lim JK, Kuo A, Di Bisceglie AM, Vargas HE, Galati JS, et al. J Hepatol. Supplement 2. Vol. 62. Vienna, Austria: 2015. All oral HCV therapy is safe and effective in patients with decompensated cirrhosis: Interim report from the HCV-target real world experience. Abstract 0007.] 50th Annual Meeting of the European Association for the Study of the Liver (EASL). April 22–16; p. S193. [Google Scholar]
- 147.Verna EC, Brown RS., Jr Hepatitis C virus and liver transplantation. Clin Liver Dis. 2006;10:919–40. doi: 10.1016/j.cld.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 148.Wright TL, Donegan E, Hsu HH, Ferrell L, Lake JR, Kim M, et al. Recurrent and acquired hepatitis C viral infection in liver transplant recipients. Gastroenterology. 1992;103:317–22. doi: 10.1016/0016-5085(92)91129-r. [DOI] [PubMed] [Google Scholar]
- 149.Dickson RC, Caldwell SH, Ishitani MB, Lau JY, Driscoll CJ, Stevenson WC, et al. Clinical and histologic patterns of early graft failure due to recurrnet hepatitis C in four patients after liver transplantation. Transplantation. 1996;61:701–5. doi: 10.1097/00007890-199603150-00005. [DOI] [PubMed] [Google Scholar]
- 150.Berenguer M. Natural history of recurrent hepatitis C. Liver Transpl. 2002;8(10 Suppl 1):S14–8. doi: 10.1053/jlts.2002.35781. [DOI] [PubMed] [Google Scholar]
- 151.Berenguer M. What determines the natural history of recurrent hepatitis C after liver transplantation? J Hepatol. 2005;42:448–56. doi: 10.1016/j.jhep.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 152.Russo FP, Zanetto A, Burra P. Timing for treatment of HCV recurrence after liver transplantation: The earlier the better. Transpl Int. 2016;29:694–7. doi: 10.1111/tri.12739. [DOI] [PubMed] [Google Scholar]
- 153.Kwo PY, Mantry PS, Coakley E, Te HS, Vargas HE, Brown R, Jr, et al. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med. 2014;371:2375–82. doi: 10.1056/NEJMoa1408921. [DOI] [PubMed] [Google Scholar]
- 154.Pungpapong S, Aqel B, Leise M, Werner KT, Murphy JL, Henry TM, et al. Multicenter experience using simeprevir and sofosbuvir with or without ribavirin to treat hepatitis C genotype 1 after liver transplant. Hepatology. 2015;61:1880–6. doi: 10.1002/hep.27770. [DOI] [PubMed] [Google Scholar]
- 155.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez- García J, Lazzarin A, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–50. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 156.Thomas DL, Shih JW, Alter HJ, Vlahov D, Cohn S, Hoover DR, et al. Effect of human immunodeficiency virus on hepatitis C virus infection among injecting drug users. J Infect Dis. 1996;174:690–5. doi: 10.1093/infdis/174.4.690. [DOI] [PubMed] [Google Scholar]
- 157.Sulkowski MS, Naggie S, Lalezari J, Fessel WJ, Mounzer K, Shuhart M, et al. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA. 2014;312:353–61. doi: 10.1001/jama.2014.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Khatri A, Wang T, Wang H, Podsadecki T, Trinh R, Awni W, et al. Washington, DC: 2014. Drug-drug interactions of the direct-acting antiviral regimen of ABT- 450/r, ombitasvir, and dasabuvir with emtricabine1tenofovir, ralte- gravir, rilpivirine, and efavirenz [Abstract]. Proceedings of the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); September 5-9. Abstract V-483. [Google Scholar]
- 159.Osinusi A, Townsend K, Kohli A, Nelson A, Seamon C, Meissner EG, et al. Virologic response following combined ledipasvir and sofosbuvir administration in patients with HCV genotype 1 and HIV co- infection. JAMA. 2015;31:1232–9. doi: 10.1001/jama.2015.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sulkowski MS, Eron JJ, Wyles D, Trinh R, Lalezari J, Wang C, et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and riba- virin for hepatitis C in patients co-infected with HIV-1: A randomized trial. JAMA. 2015;313:1223–31. doi: 10.1001/jama.2015.1328. [DOI] [PubMed] [Google Scholar]
- 161.Potthoff A, Berg T, Wedemeyer H. Late hepatitis B virus relapse in patients co-infected with hepatitis B virus and hepatitis C virus after antiviral treatment with pegylated interferon-a2b and ribavirin. Scand J Gastroenterol. 2009;44:1487–90. doi: 10.3109/00365520903329585. [DOI] [PubMed] [Google Scholar]
- 162.Rostaing L, Chatelut E, Payen JL, Izopet J, Thalamas C, Ton-That H, et al. Pharmacokinetics of alphaIFN-2b in chronic hepatitis C virus patients undergoing chronic hemodialysis or with normal renal function: Clinical implications. J Am Soc Nephrol. 1998;9:2344–8. doi: 10.1681/ASN.V9122344. [DOI] [PubMed] [Google Scholar]
- 163.Bruchfeld A, Lindahl K, Reichard O, Carlsson T, Schvarcz R. Pegylated interferon and ribavirin treatment for hepatitis C in haemodialysis patients. J Viral Hepat. 2006;13:316–21. doi: 10.1111/j.1365-2893.2005.00680.x. [DOI] [PubMed] [Google Scholar]
- 164.Pockros PJ, Reddy KR, Mantry PS, Cohen E, Bennett M, Sulkowski MS, et al. Vienna, Austria: 2015. Safety of ombitasvir/paritaprevir/ritonavir plus dasabuvir for treating HCV GT1 infection in patients with severe renal impairment or end-stage renal disease: The RUBY-1 study [Abstract]. Proceedings of the 50th Annual Meeting of the European Association for the Study of the Liver (EASL); April 22–26. Abstract LO1. [Google Scholar]
- 165.Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H, Jr, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): A combination phase 3 study. Lancet. 2015;386:1537–45. doi: 10.1016/S0140-6736(15)00349-9. [DOI] [PubMed] [Google Scholar]
- 166.Scott DR, Wong JK, Spicer TS, Dent H, Mensah FK, McDonald S, et al. Adverse impact of hepatitis C virus infection on renal replacement therapy and renal transplant patients in Australia and New Zealand. Transplantation. 2010;90:1165–71. doi: 10.1097/TP.0b013e3181f92548. [DOI] [PubMed] [Google Scholar]
- 167.Gane E, Pilmore H. Management of chronic viral hepatitis before and after renal transplantation. Transplantation. 2002;74:427–37. doi: 10.1097/00007890-200208270-00001. [DOI] [PubMed] [Google Scholar]
- 168.Dore MP, Fattovich G, Sepulveda AR, Realdi G. Cryoglobulinemia related to hepatitis C virus infection. Dig Dis Sci. 2007;52:897–907. doi: 10.1007/s10620-006-9510-9. [DOI] [PubMed] [Google Scholar]
- 169.Zuckerman E, Keren D, Slobodin G, Rosner I, Rozenbaum M, Toubi E, et al. Treatment of refractory, symptomatic, hepatitis C virus related mixed cryoglobulinaemia with ribavirin and interferon-alpha. J Rheumatol. 2000;27:2172–8. [PubMed] [Google Scholar]
- 170.Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, Egan JJ, et al. International guidelines for the selection of lung transplant candidates: 2006 update-A consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–55. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 171.Safdar K, Schiff ER. Alcohol and hepatitis C. Semin Liver Dis. 2004;24:305–15. doi: 10.1055/s-2004-832942. [DOI] [PubMed] [Google Scholar]
- 172.Anand BS, Currie S, Dieperink E, Bin EJ, Shen H, Ho SB, et al. Alcohol use and treatment of hepatitis C virus: Results of a national multicenter study. Gastroenterology. 2006;130:1607–16. doi: 10.1053/j.gastro.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 173.Grebely J, Raffa JD, Lai C, Kerr T, Fischer B, Krajden M, et al. Impact of hepatitis C virus infection on all-cause and liver-related mortality in a large community-based cohort of inner city residents. J Viral Hepat. 2011;18:32–41. doi: 10.1111/j.1365-2893.2010.01279.x. [DOI] [PubMed] [Google Scholar]
- 174.Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: Results of systematic reviews. Lancet. 2011;378:571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Edlin BR. Prevention and treatment of hepatitis C in injection drug users. Hepatology. 2002;36:S210–9. doi: 10.1053/jhep.2002.36809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Martin NK, Vickerman P, Miners A, Foster GR, Hutchinson SJ, Goldberg DJ, et al. Cost-effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology. 2012;55:49–57. doi: 10.1002/hep.24656. [DOI] [PubMed] [Google Scholar]
- 177.Lalezari J, Sullivan JG, Varunok P, Galen E, Kowdley KV, Rustgi V, et al. Ombitasvir/paritaprevir/r and dasabuvir plus ribavirin in HCV genotype 1-infected patients on methadone or buprenorphine. J Hepatol. 2015;63:364–9. doi: 10.1016/j.jhep.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 178.van Heeswijk R, Vandevoorde A, Verboven P, Boogaerts G, De Paepe E, van Solingen-Ristea R, et al. The pharmacokinetic interaction between methadone and the investigational HCV protease inhibitor telaprevir. J Hepatol. 2011;54:S491. [Google Scholar]
- 179.Luo X, Trevejo J, Van Heeswijk R, Garg V. No significant effect of the HCV protease inhibitor telaprevir on pharmacokinetics and pharmacodynamics of buprenorphine in HCV-negative volunteers. Global Antivir J. 2011;7:116–7. [Google Scholar]
- 180.Trask PC, Esper P, Riba M, Redman B. Psychiatric Side Effects of Interferon Therapy: Prevalence, Proposed Mechanisms, and Future Directions. J Clin Oncol. 2000;18:2316–26. doi: 10.1200/JCO.2000.18.11.2316. [DOI] [PubMed] [Google Scholar]
- 181.Villano SA, Vlahov D, Nelson KE, Cohn S, Thomas DL. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology. 1999;29:908–14. doi: 10.1002/hep.510290311. [DOI] [PubMed] [Google Scholar]
- 182.Wiegand J, Jackel E, Cornberg M, Hinrichsen H, Dietrich M, Kroeger J, et al. Long-term follow-up after successful interferon therapy of acute hepatitis C. Hepatology. 2004;40:98–107. doi: 10.1002/hep.20291. [DOI] [PubMed] [Google Scholar]
- 183.Fierer DS, Dieterich DT, Mullen MP, Branch AD, Uriel AJ, Carriero DC, et al. Telaprevir in the treatment of acute hepatitis C virus infection in HIV-infected men. Clin Infect Dis. 2014;58:873–9. doi: 10.1093/cid/cit799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Camma C, Almasio P, Craxi A. Interferon as treatment for acute hepatitis C. A meta-analysis. Dig Dis Sci. 1996;41:1248–55. doi: 10.1007/BF02088245. [DOI] [PubMed] [Google Scholar]
- 185.Ghany MG, Strader DB, Thomas DL, Seeff LB. for the American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: An update. Hepatology. 2009;49:1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hofer H, Watkins-Riedel T, Janata O, Penner E, Holzmann H, Steindl-Munda P, et al. Spontaneous viral clearance in patients with acute hepatitis C can be predicted by repeated measurements of serum viral load. Hepatology. 2003;37:60–4. doi: 10.1053/jhep.2003.50019. [DOI] [PubMed] [Google Scholar]
- 187.Basu PP. Sofosbuvir and Ledipasvir versus Sofosbuvir and Simeprevir combination therapy in the management of acute hepatitis C: A randomized open label prospective clinical pilot study. AASLD. 2015 Abstract 1074. [Google Scholar]


