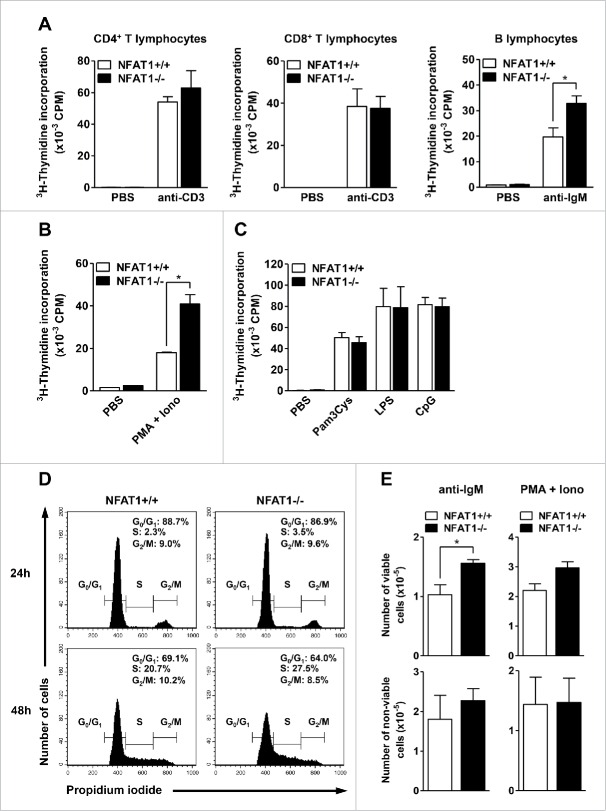
Figure 2.
NFAT1 controls cell proliferation in primary B lymphocytes. (A) CD4+, CD8+ T lymphocytes, and B lymphocytes were purified from naive NFAT1+/+ and NFAT1−/− mice by negative selection with magnetic beads. Cells were left unstimulated (PBS) or stimulated in vitro with plate-bound anti-CD3 (1 μg/mL) or anti-IgM (5 μg/mL) for 36 hours. (B and C) Purified B lymphocytes from naive NFAT1+/+ and NFAT1−/− mice were left unstimulated (PBS) or stimulated in vitro with PMA (10 nM) and Ionomycin (1 μM) (B) or Pam3Cys (0.1 μg/mL), LPS (1 μg/mL), and CpG (1 μg/mL) (C) for 36 hours. (A-C) After stimulation, cells were pulsed with 3H-thymidine (5 μCi/mL) for 8 hours. Cells were then harvested and 3H-thymidine incorporation was analyzed by β-spectrometer. CPM refers to counts per minute. (D and E) Purified B lymphocytes from naive NFAT1+/+ and NFAT1−/− mice were stimulated with PMA (10 nM) and Ionomycin (1 μM) or anti-IgM (5 μg/mL) as indicated. (D) Cells were stimulated with PMA/Ionomycin for the indicated time points, labeled with propidium iodide, and DNA content was analyzed by flow cytometry for cell cycle phases. (E) Cells were stimulated with anti-IgM (left panels) or PMA/Ionomycin (right panels) for 24 hours, and the numbers of viable (upper panels) and non-viable (lower panels) cells were assessed with Trypan Blue using a Neubauer chamber. All results were obtained from a pool of 3 mice, and are representative of at least 3 independent experiments. Results are expressed as mean and error bars represent SEM. Asterisks indicate significance levels compared to controls (p < 0.05).