ABSTRACT
Primary cancer resections and in selected cases surgical metastasectomies significantly improve survival, however many patients develop recurrences. Circulating tumor cells (CTCs) function as an independent marker that could be used in the prognostication of different cancers. Sampling of blood and bone marrow compartments during cancer resections is a unique opportunity to increase individual tumor cell capture efficiency. This review will address the diagnostic and therapeutic potentials of perioperative tumor isolation and highlight the focus of future studies on characterization of single disseminated cancer cells to identify targets for molecular therapy and immune escape mechanisms.
KEYWORDS: Circulating tumor cells, disseminated tumor cells, metastasectomy, surgery
Introduction
There have been very different and often spiritual theories since antique times about the development and spread of cancers. In 1889, the British surgeon Stephen Paget suggested the ´seed and soil´ theory of metastasis of cancer allowing tumor cells to seed via the bloodstream into distant organs (the ´soils´).1-3 This concept has been widely accepted until the 21st century. The American surgeon William Halsted (born 1852) extended this theory to the lymphatic system and integrated it into his practice of breast cancer by performing resections of axillary lymph nodes.4
The initial morphological description of circulating tumor cells (CTCs) goes back to 1869 when the Australian physician Thomas Ashworth identified cancer cells similar to the ones of the primary tumor in the blood vessels of autopsied cancer patients.5 Since then a number of techniques have been developed for the isolation of CTCs in peripheral blood, including reverse transcriptase polymerase chain reaction (rt-PCR), immunocytochemistry, flow cytometry, microchips, and size-based filtration methods.6 Enrichment and detection of CTC in the blood has been one of the most active areas in translational cancer research. More than 50 detection assays have been established with more than 200 clinical trials being incorporated CTC.7
Most trials have incorporated patients with advanced stage IV disease since these patients are harboring higher numbers of CTCs than patients at the early stages.8-10 Surgical candidates with localized, resectable cancers and limited tumor burden are a specific set of potentially curable patients. These patients integrating with additional systemic treatments before or after surgical resection could further improve survival rates. But in patients with limited metastases that usually have low baseline CTC numbers perioperative CTC isolation is a unique opportunity to increase CTC yield.11 Intraoperative CTC isolation is an exceptional opportunity to isolate more CTCs as it allows access to blood in proximity to the tumor outflow. The major advantage for surgeons is that they often have access to compartments that other disciplines do not have, e. g. tumor blood outflow and inflow in proximity to the tumor. CTC dissemination can also be studied right before and after resection at any time point. Moreover, the surgical technique and extent of manipulation may impact CTC shedding, recurrence rates and eventually the outcome of a cancer patient. Individual cancer cells that have spread to other organ sites, such as the bone marrow (BM), are called disseminated tumor cells (DTCs). Although DTCs are present in other compartments including the lymphatic system, BM might serve as a special reservoir for DTCs, where they can home and survive and then recirculate to invade other distant organs such as liver or lungs, which might offer more favorable growth conditions. The bone marrow might be a reservoir for blood-borne DTCs.
Clinically, development of objective criteria for surgery selection, prognosis and multidisciplinary treatments in cancer patients has been a challenging task. The ability to isolate CTCs provides a powerful tool to monitor the response to treatment, improve early detection and personalize prognosis for our patients. 12 Detection and characterization of CTCs could provide valuable insight toward improving treatment and identifying novel biologic targets.
Perioperative detection of circulating tumor cells
CTC detection techniques
One of the major limitations in CTC detection is rare CTC quantity in the peripheral blood.13,14 One milliliter of human blood carries approximately one billion red blood cells, 7 million white blood cells and 300 million platelets, but only about 1–10 CTCs.15 The most effective CTC detection test would have an optimal (100%) sensitivity, specificity, positive/negative predictive value and overall accuracy. The detected CTCs should be the ones that have the potential to grow to a solid metastasis and could be replicated in culture so that features of malignancy and therapeutic chemosensitivity testing can be reliably done. There have been different types of CTCs described. The traditional CTC has been described to be large with rather irregular shape and subcellular morphology with an intact, viable nucleus, expressing cytokeratins (CK) confirming the epithelial origin and does not express CD45 excluding a haematopoietic origin.16 However, there have been CK negative (CK-) CTCs described that might be the ones of highest relevance that express mesenchymal and stem cell markers.17 There are also apoptotic CTCs with features of cells undergoing cell death. Yet, small CTCs that are CK+ and CD45- resembling rather white blood cells could also be of high relevance, in particular since small cell cancers are highly aggressive and dedifferentiated tumors. Finally, clusters of 2 or more CTCs that are adherent to each other are a quite often noted and interesting phenomenon that have been associated with poor prognosis.18,19
Only a few (1/1000) CTCs have the potential to eventually initiate metastatic growth.13,14 Most CTCs are non-proliferating (Ki-67 negative) and resistant to chemotherapy, and some subsets may just have a promotor role for other cancer cells.20 Multiple techniques for CTC enrichment and detection have been developed.6 Quite many CTC capturing method are based upon immuno-affinity relying on the expression of epithelial cell surface markers such as EpCAM-1. Other methods depend on on biophysical characteristics that distinguish cancer cells from normal cells. As outlined in Table 1, direct CTC detection methods are also based on cell size (e. g., ISET®, FSMA/microfilters), deformability, bioelectric properties, or immune-affinity (magnetic cell sorting (MACS), immunomagnetic enrichment techniques (e. g., CellSearch®)).21 Gradient separation centrifugation with Ficoll-Paque or OncoQuick® has been used to separate CTCs in high yield based on their Bouyant density. In addition, cell adhesion matrix platforms, enzymatic (EPISPOT), electrophoresis, or microscopic features have also been reported.21
Table 1.
Examples of detection technologies for CTCs in peripheral blood and DTCs in bone marrow.
| Enrichment approach | Technology | Source | Primary enrichment | Secondary CTC/DTC detection technique and identification markers | Comments |
|---|---|---|---|---|---|
| Immunological | CellSearch® by Veridex | Blood (7.5 ml) | EpCAM-labeled ferrofluids | Immunocytochemistry: CK (8/18/19), CD45, DAPI (nuclear staining) | Semiautomated system; FDA-approved for stage IV breast, colon and prostate cancers |
| AdnaTest® | Blood (7 ml) | Immunomagnetic (EpCAM, MUC-1, Her2) | Multiplex PCR: Twist, Akt2, PI3Kα | Detection of epithelial, mesenchymal and stem cell features | |
| EPISPOT assay | Blood, BM (10 ml) | Immunomagnetic (depletion of CD45- cells) | Culture & Immunocytochemistry: CK19, MUC1, Cath-D (breast); CK19 (colon); PSA (prostate); TG (thyroid) | Detection of viable, secreting CTCs/DTCs | |
| PowerMag® | Blood (5 ml) | Dextran-coated magnetic nanoparticles: depletion of CD45+ cells | Immunocytochemistry: EpCAM | Detection technique is comparable to FDA-approved CellSearch® system | |
| Size | ISET® (Isolation by Size of Tumor cells) | Blood (5 ml) | Filter | Cytology & Immunocytochemistry: haematoxylin, CK | High sensitivity |
| FSMA (flexible spring microarray) | Blood (7.5 ml) | 3-dimensional microfilter | Immunocytochemistry: CK | High sensitivity, mesenchymal and stem cell markers can be stained, allows culturing | |
| Density | Oncoquick® | Blood (15 ml) | Density gradient centrifugation | Immunocytochemistry: CK | Allows different additional analysis (immunocytochemistry, rt-PCR, cell culture, cell sorting) |
| Ficoll-Paque | BM (15 ml) | Density gradient centrifugation | Immunocytochemistry: CK | Traditional technique for purification of DTCs from bone marrow | |
| Electrophoresis | ApoStream® | Blood (7.5 ml) | Dielectrophoretic forces | Immunocytochemistry: EpCAM, CK | Cells can be cultured |
| Microscopy | Epic Sciences® | Blood (10 ml) | RBC lysis, centrifugation | Immunocytochemistry: CK | Automated scanning system, EpCAM-independent, CTCs are identified on marker expression and morphology |
BM: bone marrow; EpCAM: Epithelial cell adhesion molecule; CK: Cytokeratin; RBC: Red blood cell.
The only technology approved by the US Food and Drug Adminstration (FDA) is CellSearch® by Veridex. It utilizes several molecular parameters to isolate CTCs: antibody-mediated immunomagnetic enrichment with epithelial cell adhesion molecule (EpCAM), nuclear staining with 4′,6-diamidino-2-phenylindole (DAPI), and immunofluorescence detection with cytokeratins (CKs) and CD45.22 CellSearch® qualifies a cell as a CTC if it is EpCAM+, CK+, has an evident nucleus visualized by DAPI, and is negative for the pan-leucocyte marker CD45. Due to its proven reliability and prognostic impact, it is the only system approved by the FDA for the enumeration of CTCs in metastatic colorectal, prostate, and breast cancers.23 Several studies have demonstrated the clinical significance of CTC numbers as a prognostic marker in these tumors.6,23-26
However, the CellSearch® technology does not detect CTCs in many patients with widely metastatic disease, even some of them have exceedingly high CTC numbers in the peripheral blood.6 Therefore, CTCs were also isolated by filtration from the waste of CellSearch system, indicating that cells are missed by the CellSearch® system.27 EpCAM-independent detection techniques have a higher CTC capture efficiency than CellSearch®.19 In most carcinomas, tumor progression implicates a shift toward a mesenchymal phenotype, a process referred to as the epithelial-mesenchymal transition (EMT) and considered to be crucial for metastasis.28-30 EpCAM is not expressed by all tumors and can be downregulated during EMT.31 EpCAM-independent isolation techniques detect more CTCs, even if the primary tumor or metastasis expresses EpCAM at high levels.9,11 These findings suggest that a subset of CTCs may still down-regulate EpCAM and escape the CellSearch® system, and that may explain why higher CTC numbers could be detected with the EpCAM independent methods.11 Size-based detection techniques have also been developed since CTCs (12–25 µm) are larger than 95% of all leukocytes (7–15 µm) and much larger than erythrocytes (5–7 µm).19,32 Filter devices can be microfabricated from polymers.33 These devices allow isolation of CTCs using the CellSearch® system's definition of CTCs (Pan-CK+/CD45-/DAPI+), yet they are EpCAM-independent. Our previous studies have shown that a filter device has a higher CTC capture efficiency than CellSearch®.19,34 Microfiltration also allows rapid bedside processing of blood and these devices have been widely tested for cell enrichment.33,35-39 Rapid enrichment is desirable to minimize disruption to cells and to preserve cell phenotypes. These and other techniques give option to add additional, more specific markers and culture CTCs.19,40
Perioperative CTC detection
A significant advantage of CTC isolation in a surgical setting is the access to compartments that are usually not accessible, e. g. tumor blood outflow and inflow in proximity to a tumor (Table 2). CTC dissemination can also be studied during, right before and after resection or at any other time point. There have been few studies published on perioperative detection of CTCs, and most of these have involved low patient numbers. In addition to major and seminal studies on CTCs in advanced/stage IV breast cancer, one study demonstrated presence of CTCs in 30% of patients with localized breast cancer both before and after surgery, with a shift from positive to negative and vice versa in 40% of cases.24,41 In colorectal cancer, the detection rate and quantity of CTC is significantly increased intraoperatively during colorectal cancer resection and is significantly higher in the sampled portal vein compared to peripheral venous blood.42 Less CTC were also detected during minimally invasive laparoscopic surgery compared to open approach, most likely as result of the medial to lateral approach that ligated tumor supplying vessels early.42 In addition, studies have included liver resections for colorectal cancer metastases.43 In one trial the peripheral vein, an artery, the hepatic portal vein, and the hepatic vein were sampled and the study demonstrated a significant higher number in the portohepatic blood circulation and a significant CTC decrease after cancer removal.44,45 Other studies with few patients demonstrated that CTCs can be isolated more frequently in tumor outflow than in the peripheral blood during surgery of primary colorectal cancer.44,46 A recent trial revealed that CTCs are elevated in the outflow (mesenteric vein) of primary colorectal cancer, and comparison with peripheral blood demonstrated lower numbers of CTCs, suggesting that the liver captures CTCs before they enter the peripheral circulation.43 Of note, the CTC yield is much higher during colorectal cancer lung and liver metastasectomy than preoperatively.11 But investigators have also integrated the concept of perioperative cancer outflow CTC detection in primary lung cancer. A lung lobe has a large and single outflow vessel, the pulmonary vein that can be sampled intraoperatively at low risk. Pulmonary vein sampling results in significantly higher yields of CTCs in early-stage lung cancers.47 CTC detection rate has been demonstrated to be much higher after surgical manipulation in particular in lung cancers with lymphatic invasion.48 CTCs were seen in less higher numbers in patients treated with minimally invasive, video-assisted (VATS) lobectomy than in cases that underwent open thoracotomy.49
Table 2.
Different compartments that can be sampled for perioperative CTC and DTC isolation.
| CTC sampling sites | Compartment | Sampling time points | |
|---|---|---|---|
| Abdominal cancers | Peripheral vein | Outflow (posthepatic) | Anytime |
| Portal/mesenteric vein | Outflow (prehepatic) | Surgery | |
| Radial artery | Inflow | Surgery | |
| Central venous line | Outflow (posthepatic) | Surgery | |
| Mediport | Outflow | Anytimet | |
| Lung cancer | Peripheral vein | Inflow | Anytime |
| Central venous line | Inflow | Surgery | |
| Mediport | Inflow | Anytime | |
| Pulmonary vein | Outflow (proximal) | Surgery | |
| Radial artery | Outflow (distal) | ||
| Other cancers (e. g., breast, prostate, head and neck) | Peripheral vein | Inflow | Anytime |
| Radial artery | Inflow | Surgery | |
| Central venous line | Outflow | Surgery | |
| Mediport | Outflow | Anytime | |
| DTC sampling sites | |||
| Lung cancer |
Sternum, ribs, iliac crest |
Bone marrow |
Surgery |
| All cancers | Iliac crest | Bone marrow | Surgery |
| Lymph nodes | Outflow of lymphatic system/first draining (sentinel) lymph node | Surgery |
To what extent the surgical technique and manipulation may impact CTC shedding, recurrence rates and eventually the outcome of cancer patients has still to be investigated. The impact of ischemia (after ligation of supplying blood vessels) on tumor cell dissemination during cancer surgery could also be of high interest to the field. A study on transarterial chemoembolization (TACE) of hepatocellular cancer has been performed and there were more CTCs in central right atrial than in peripheral venous blood, yet the CTC quantity remained unchanged after TACE at both sample sites.50
There are also other interesting areas that can be addressed in future, such as studying responders undergoing neoadjuvant treatment protocols. A critical issue is not only the compartment, but also the time point for blood draws. In own studies we chose the skin incision (baseline), resection phase (during manipulation or before ligation of major tumor-supplying vessels), shortly (e. g., 30 minutes) after removal of specimen and postoperative day one.11 During the resection phase CTC yield is highest. Other time points in the long term for longitudinal outcome studies after surgery might be very valuable, also to test how long CTCs do persist in the blood and when systemic treatments might be most appropriate to target these. Just to mention, in the perioperative setting blood transfusions might exclusion criteria for perioperative CTC analysis.
How CTCs are shedded (constantly, pulsatile, randomly, purely mechanical) is also unknown. Moreover, whether these cells are viable, apoptotic or necrotic cancer cells has also to be further tested. The efficiency of perioperative detection actually raises a potential concern, since there are now a number of reports which describe physical translocation of CTCs into the circulation (blood or lymph) during surgical procedures.51-56 In some instances, their forced release appears to have prognostic relevance for patients. For example, hematogenous dissemination of cancer cells during surgery for lung cancer has been reported and was related to clinical prognosis, and similar observations have been reported for hematogenous and lymphatic spread in esophageal, stomach and colorectal cancers.54,55,57-59 On the other hand, in other cancers (e. g, pancreatic) the forced mechanical dissemination does not affect prognosis.60 There are also reports of direct physical seeding of cancer cells during percutaneous biopsies.61,62 Of note is that CTC clusters (consisting of 2 to up to 20 adherent cells) from clinical blood samples have been described and that they can be preserved for analysis by gentle handling and processing of the samples.19 Aggregate clusters of CTCs have been reported in several cancer types, but their clinical significance is not fully understood.39,63-68 Their exact biologic role in tumor spread applying novel technologies has still to be investigated. Clotting of perioperative blood samples can be problematic for investigators. This could most likely be due to hypercoagulable states in cancer patients and risk for thrombotic events is particularly high during cancer surgery with challenging perioperative fluid management.69,70 Finally, it has been demonstrated in mice that CTCs might relocate to the primary cancer organ site (´tumor self-seeding´) and promote tumor progression.71 This self-seeding of CTCs could explain local recurrences in the original organ despite complete surgical excision.
Disseminated tumor cells (DTCs) in the bone marrow
Individual tumor cells migrating into the bone marrow (BM), lymphatic system and other organs except the blood, are called disseminated tumor cells (DTCs). DTCs can be consistently detected in the BM in all solid tumor types that do not have distant metastases (M0) at a rate of 20 to 60% (Table 3).72 Although most data on the prognostic value of DTCs are for breast cancer, studies from several independent institutions on patients with colon, esophageal, lung and prostate cancer demonstrate the association between presence of DTCs at primary surgery for localized cancer with subsequent metastatic relapse.73-76 Detection of residual disease by repeated BM sampling is the standard of care in patients with leukemia or lymphoma, but invasive BM sampling is not yet accepted and difficult to introduce in the clinical management of patients with solid tumors, although BM sampling can be done without pain or morbidity perioperatively when patients are under anesthesia for tumor resections.75,76
Table 3.
Detection rates of CTCs in peripheral blood and DTCs in bone marrow of patients without distant metastases (M0).
| Cancer type | CTC detection rate | References | DTC detection rate | References |
|---|---|---|---|---|
| Breast | 25–40 % | 41, 94, 95 | 20–40 % | 73, 75 |
| Colorectal | 5–50 % | 42, 96 | 20–30 % | 85, 97, 98 |
| Esophageal | 20 % | 99 | 30–40 % | 76, 100 |
| Gastric | 30–60 % | 101, 102 | 35–60 % | 103, 104 |
| Head and neck | 40–80 % | 105, 106 | 20–30 % | 107, 108 |
| Lung (NSCLC) | 30–50 % | 109, 110 | 40–60 % | 111-113 |
| Pancreatic | 10–75 % | 114, 115 | 20–35 % | 97, 116, 117 |
| Prostate | 10–98 % | 118, 119 | 20–50 % | 120-122 |
Although DTCs are present in other compartments including the lymphatic system, BM might serve as a special reservoir for DTCs. DTCs can home to the BM and survive and then recirculate to invade other distant organs such as the liver or lungs that might offer optimal growth environments. The BM might even serve as a reservoir for CTCs in the peripheral blood. Subsets of DTCs and CTCs share phenotypes with breast cancer stem cells (e.g. CD44+ CD24–/low).77 DTCs could use the BM stem-cell environment as a niche to survive and persist in a dormant state over years or even decades. Understanding the stage of dormancy and the initiators to allowing dormant DTCs to get reactivated, grow or spread and to identify the ´stem cell´ of macrometastases are some of the most critical areas of translational research on tumor cell dissemination and biology.
Cancer cells expressing chemokine receptors might get attracted to the BM (or other organs) by specific chemokines that are excreted from cells in metastatic target organs. The BM homing receptor for lymphocytes is the chemokine receptor CXCR4 (interacting with the chemokine SDF-1α) that has been shown to be associated with migration of cancer cells to the BM in humans and mice.76,78
Although BM analyses provide important information about the biology of cancer metastasis, peripheral blood drawings are more acceptable in the clinical management of carcinoma patients than invasive BM aspirations. Most research groups are, therefore, focusing on the clinical value of CTC analyses, in particular for the real-time monitoring of the efficacy of systemic therapies and the detection of molecular targets related to drug sensitivity or resistance. Yet, the BM might be a critical compartment site carrying a key role in cancer spread.
Phenotype and genotype analysis
Trial results from patients with advanced and metastatic cancers reveal the value of CTC detection as a ‘liquid biopsy', but studies on cancer patients at earlier and resectable stages are not so promising as they have low CTC counts.9 Yet, these early stage patients are clinically very interesting as they are potentially curable and can develop recurrence at later time points suggesting that they harbor disseminated cancer cells. Current CTC detection technologies based on EpCAM selection miss a substantial number of CTCs and these CTCs might have an aggressive phenotype such as an immune cell or mesenchymal phenotype with stem cell features. In most carcinomas, tumor progression implicates an EMT shift considered to be crucial for metastasis.28-30 Mesenchymal-like CTCs present in the blood of carcinoma patients are likely to be missed with CellSearch® as CTC detection depends on EpCAM expression. However, CTCs without EpCAM expression may have a significant role in developing distant metastasis.79,80 In previous studies we and other investigators were able to isolate CTCs from cancer patients with a mesenchymal phenotype expressing vimentin.81 It has been observed that CTCs often stained positively for both epithelial markers (CKs) and the mesenchymal cell marker vimentin (CK+/vimentin+/CD45-).19 Expression of vimentin intermediate filaments and downregulation of epithelial cell markers is implicated in EMT, which is considered a pre-requisite for CTC dissemination.66,82-84 Other molecules involved in cell adhesion, migration and chemotaxis have also been described to have prognostic impact and potential key roles in metastatic dissemination of single cancer cells to the lymph nodes and bone marrow.76,85
It will be critical to identify markers for CTCs with an EMT phenotype that can distinguish epithelial tumor cells from normal mesenchymal blood cells. Moreover, robust multiplex technologies for detailed molecular analyses of CTCs need to be developed and validated in clinical trials. Finally, the identification of the metastatic founder cells among CTCs might require xenotransplantation studies in immunodeficient mice. Genomic and protein or mRNA analysis can be done by single-cell gene amplification, FISH or immunocytochemistry. Molecular characterization of single CTCs in the same patient demonstrated heterogeneity of EGFR expression and genetic alterations in EGFR, KRAS, and PIK3CA, possibly explaining the variable response rates to EGFR inhibition in patients with colorectal cancer.86,87 Moreover, genetic testing of CTCs will also gather more understanding on cancer biology. Several specific and activating gene mutations (e. g., EGFR) or gene amplifications (e. g., HER2) can be used to individualize treatments in lung or breast cancer with innovative humanized antibody treatments.88
Culturing, chemosensitivity testing, mouse models
Culturing of CTCs from the peripheral blood can yield most important information on the biology of replicating cancer cells and aid in in vivo and in vitro testing for personalized clinical treatment strategies (Fig. 3). However, culturing of CTCs (and DTCs) is challenging as most of these cells do not have the ability to replicate and if so, an appropriate environment has to be simulated in vitro or in vivo. It has been estimated that about 2.5% of CTCs cause micrometastases and 0.1% of CTCs have the potential to grow to a solid macrometastasis.13,14,89 In a recent study colorectal cancer cell lines were successfully grown from peripheral blood of patients.90 Functional studies showed that these cultured cells were even capable of growing into in vivo tumors after xenografting in immunodeficient mice. The establishment of cancer cell lines from CTCs will allow functional studies on the biology of CTCs as well as in vitro and in vivo chemosensitivity testing. OncoQuick® enrichment is based on the fact that CTCs have a lighter Bouyant density than peripheral blood mononuclear cells, so that they remain on top of the liquid (of defined density) used for the separation after centrifugation. We have previously reported on colorectal cancer CK/CD45+ CTCs expressing the pan-macrophage marker CD14, a potential fusion cell that can influence the antigen recognition of the host immune system to the advantage of the cancer.40
Figure 1.
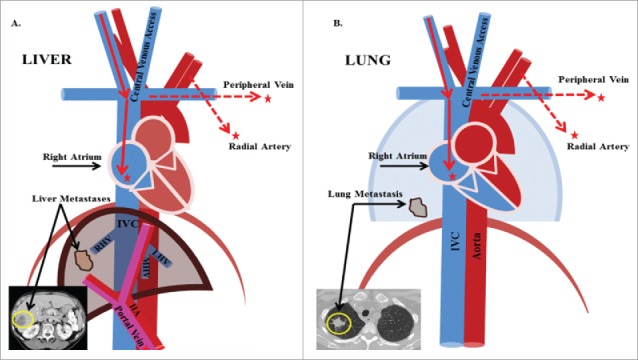
Blood sampling access sites for perioperative CTC detection. During major surgeries, such as liver and lung resections, routinely placed access catheters in the central venous system and radial artery can be used for blood draws at no additional risks. The tumor outflow of liver cancers are the hepatic veins followed by the inferior vena cava and right atrium. Blood from the latter is accessible via the central venous line. The blood outflow of the lung as the oxygenating organ is the pulmonary vein that can be punctured intraoperatively, but the radial artery represents it more distal and is accessible without additional intervention.
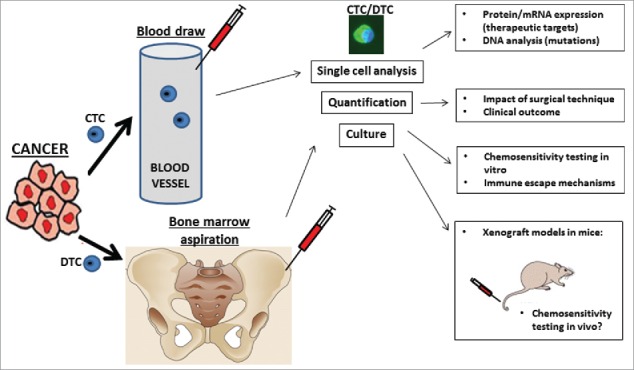
Figure 3.
Current and future potential clinical implications of perioperative CTC and DTC detection.
Growing into in vivo tumors from cultured CTCs or DTCs after xenografting in immunodeficient mice has been achieved by few investigators only, and this holds outstanding potential for individual drug testing.90
Immune escape mechanisms and induction of immune tolerance: fusion cells
In 1911 the German pathologist Otto Aichel (1871–1935) suggested already that cancer cells fuse with white blood cells to hybrids allowing them to migrate in the blood and lymphatic system and eventually grow to metastases.91 Consequently tumor cell fusion with white blood cells (‘hybrids’) has been a long-standing theory of cancer spread.92 More recently, different studies suggested macrophage–tumor cell fusions (´hybrids´) in cancer spread, including colorectal cancer and melanoma.92 Tumor-associated macrophages may fuse with epithelial cancer cells at the site of the primary tumor or even metastasis. These hybrids might then induce the EMT transition in some cancer cells, allowing them to escape into the blood and lymphatic system (along with the hybrid cells) and colonize distant organs.40,92 Like other investigators we hypothesize that CTCs undergo EMT and acquire the skill to induce immune tolerance. CD45 is a pan-leucocyte marker and CD14 is highly expressed by macrophages. In a previous study we detected large CK+/CD45+/CD14+ CTCs enriched by an established and simple centrifugation technique (OncoQuick®) and cultured CTCs from melanoma, pancreatic and colorectal patients.40 These cultured CTCs from peripheral blood of cancer patients (but not healthy controls) could be grown through multiple passages. Further analysis of viable and replicating CTCs in culture revealed again CK positive CTCs expressing leucocyte marker CD45 and macrophage marker CD14 supporting the hypothesis that certain CTCs fuse with macrophages. This would allow CTCs/macrophage hybrids to induce T cell immune tolerance (Fig. 2). A recent study on CTCs in colorectal cancer also included a gene expression analysis that revealed both a pronounced upregulation of CD47 as a potential immune-escape mechanism and a significant downregulation of several other pathways, suggesting a dormant state of viable CTCs.93 Results suggested mutational heterogeneity between tumor tissue and CTCs and upregulation of immune-escape pathways that may be responsible for survival of CTCs in cancer patients. Further emphasis on analysis of cultured CTCs that are capable to replicate could explain what kind of metastases cause CTC dissemination, and these studies can lead to the detection of novel molecular therapy targets and establishment of immune therapy modalities.
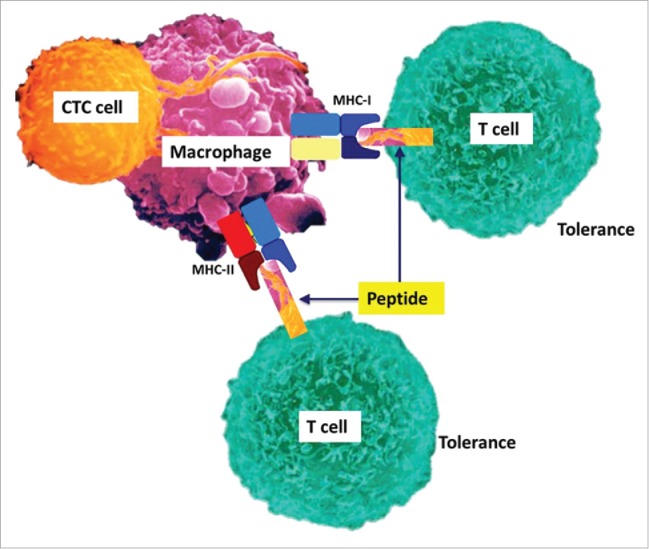
Figure 2.
Concept of a hybrid cell after fusion of a CTC with macrophages to induce immune tolerance. CTC culturing from melanoma and colorectal cancer patients with a density centrifugation technique revealed CK+ CTCs that express pan-macrophage marker CD14 and leucocyte marker CD45. It can be hypothesized that CTCs capable to spread and replicate in other organs induce immune tolerance by fusing with macrophages and expressing tumor antigens to the host immune system.
Perspectives
Perioperative detection of single tumor cells in different body compartments holds outstanding potential to increase understanding of metastasis biology and improve treatment options for patients (Fig. 3). There are certainly several pitfalls with studies on perioperative detection of CTCs since they mostly low sample size and potential for selection bias. Patients are heterogeneous and some subjects have previously received various systemic and surgical treatments. On the other hand, study populations are unique as patients either have a primary tumor only or a limited number of metastases that will be completely resected and these patients have a high chance for cure.
It is of particular interest to characterize CTCs with an immune cell, stem cell and mesenchymal phenotype. The ultimate goal is to develop reliable and repeatable methods to identify and culture CTCs and examine their chemosensitivity to novel drugs and various treatments. The understanding of how cancer cells manipulate the immune system and the host environment will significantly increase the options for oncologic treatments. CTC phenotype analysis needs to be multiplexed with several molecular markers and include mutational testing. Moreover, detection of high CTC numbers in the cancer outflow could lead to clinical trials on local and even intra- or perioperative treatments to prevent further CTC dissemination, such as transarterial drug application. CTC sampling in direct proximity to the tumors in addition to routine sampling at baseline or in follow-up can be added during resections. As transjugular hepatic vein blood (outflow of the liver) sampling is part of routine preoperative evaluation, CTC detection could be added even in non-surgical candidates for therapy stratification at minimal risk. In future studies, CTC data will have to be correlated to survivorship data, and results may explain why and which CTCs cause recurrences.
CTC isolation in general and specifically during surgical resection will increase understanding of CTC shedding during surgery. Future studies will need to improve techniques for isolation, culturing and characterization of relevant CTCs to further understand cancer spread biology, and improve management of patients undergoing cancer-related surgery. In conclusion, the detection and characterization of individual tumor cells in the blood, bone marrow and other organs will provide new insights into the complex biology of cancers with important implications for the clinical management of oncologic patients.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgements
We thank all participating cancer patients for their involvement in clinical studies on tumor cell dissemination.
References
- 1.Paget S. The distribution of secondary growths in cancer of the breast. Lancet 1889:571-3; http://dx.doi.org/ 10.1016/S0140-6736(00)49915-0 [DOI] [PubMed] [Google Scholar]
- 2.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 2003; 3:453-8; PMID:12778135; http://dx.doi.org/ 10.1038/nrc1098 [DOI] [PubMed] [Google Scholar]
- 3.Hajdu SI. Thoughts about the cause of cancer. Cancer 2006; 106:1643-9; PMID:16534793; http://dx.doi.org/ 10.1002/cncr.21807 [DOI] [PubMed] [Google Scholar]
- 4.Cotlar AM, Dubose JJ, Rose DM. History of surgery for breast cancer: radical to the sublime. Curr Surg 2003; 60:329-37; PMID:14972270; http://dx.doi.org/ 10.1016/S0149-7944(02)00777-8 [DOI] [PubMed] [Google Scholar]
- 5.Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J 1869; 14:146-7. [Google Scholar]
- 6.Joosse SA, Pantel K. Biologic challenges in the detection of circulating tumor cells. Cancer Res 2013; 73:8-11; PMID:23271724; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-3422 [DOI] [PubMed] [Google Scholar]
- 7.Bednarz-Knoll N, Alix-Panabieres C, Pantel K. Clinical relevance and biology of circulating tumor cells. Breast Cancer Res 2011; 13:228; PMID:22114869; http://dx.doi.org/ 10.1186/bcr2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaifi JT, Kunkel M, Dicker DT, Joude J, Allen J, Zhu J, Yang Z, Sarwani NE, Staveley-O'Carroll KF, El-Deiry ES. Circulating tumor cell levels are elevated in colorectal cancer patients with high tumor burden in the liver. Cancer Biol Ther 2015; 16:690-8; 25785486; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaifi JT, Kunkel M, Zhu J, Dicker DT, Gusani NJ, Yang Z, Sarwani NE, Li G, Kimchi ET, Staveley-O'Carroll KF, El-Deiry WS. Circulating tumor cells are associated with diffuse spread in stage IV colorectal cancer patients. Cancer Biol Ther 2013; 14; PMID:24153154; http://dx.doi.org/ 10.4161/cbt.26884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das A, Kunkel M, Dicker DT, Joudeh J, Scicchitano A, Allen JE, Sarwani NE, Yang Z, Kaifi JT, Zhu J, et al.. Clinico-pathological significance of serial measurement of circulating tumor cells in 24 metastatic colorectal cancer patients treated with combination chemotherapy. Cancer Biol Ther 2015; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaifi JT, Kunkel M, Das A, Harouaka RA, Dicker DT, Li G, Zhu J, Clawson GA, Yang Z, Reed MF, et al.. Circulating tumor cell isolation during resection of colorectal cancer lung and liver metastases: a prospective trial with different detection techniques. Cancer Biol Ther 2015; 16:699-708; PMID:25807199; http://dx.doi.org/ 10.1080/15384047.2015.1030556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu MC, Shields PG, Warren RD, Cohen P, Wilkinson M, Ottaviano YL, Rao SB, Eng-Wong J, Seillier-Moiseiwitsch F, Noone AM, et al.. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol 2009; 27:5153-9; PMID:19752342; http://dx.doi.org/ 10.1200/JCO.2008.20.6664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lianidou ES, Mavroudis D, Sotiropoulou G, Agelaki S, Pantel K. What's new on circulating tumor cells? A meeting report. Breast Cancer Res 2010; 12:307; PMID:20727231; http://dx.doi.org/ 10.1186/bcr2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J Cell Biol 2011; 192:373-82; PMID:21300848; http://dx.doi.org/ 10.1083/jcb.201010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mockelmann N, Laban S, Pantel K, Knecht R. Circulating tumor cells in head and neck cancer: clinical impact in diagnosis and follow-up. Eur Arch Otorhinolaryngol 2014; 271:15-21; PMID:23408023; http://dx.doi.org/ 10.1007/s00405-013-2391-6 [DOI] [PubMed] [Google Scholar]
- 16.Racila E, Euhus D, Weiss AJ, Rao C, McConnell J, Terstappen LW, Uhr JW. Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci U S A 1998; 95:4589-94; PMID:9539782; http://dx.doi.org/ 10.1073/pnas.95.8.4589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrinucci D, Bethel K, Kolatkar A, Luttgen MS, Malchiodi M, Baehring F, Voigt K, Lazar D, Nieva J, Bazhenova L, et al.. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol 2012; 9:016003; PMID:22306768; http://dx.doi.org/ 10.1088/1478-3975/9/1/016003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al.. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014; 158:1110-22; PMID:25171411; http://dx.doi.org/ 10.1016/j.cell.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harouaka RA, Zhou MD, Yeh YT, Khan WJ, Das A, Liu X, Christ CC, Dicker DT, Baney TS, Kaifi JT, et al.. Flexible Micro Spring Array Device for High-Throughput Enrichment of Viable Circulating Tumor Cells. Clin Chem 2013; PMID:24132944; http://dx.doi.org/ 10.1373/clinchem.2013.206805 [DOI] [PubMed] [Google Scholar]
- 20.Muller V, Stahmann N, Riethdorf S, Rau T, Zabel T, Goetz A, Janicke F, Pantel K. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin Cancer Res 2005; 11:3678-85; PMID:15897564; http://dx.doi.org/ 10.1158/1078-0432.CCR-04-2469 [DOI] [PubMed] [Google Scholar]
- 21.Friedlander TW, Premasekharan G, Paris PL. Looking back, to the future of circulating tumor cells. Pharmacol Ther 2013; 142:271-80; PMID:24362084; http://dx.doi.org/ 10.1016/j.pharmthera.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 22.Hayes DF, Smerage JB. Circulating tumor cells. Prog Mol Biol Transl Sci 2010; 95:95-112; PMID:21075330; http://dx.doi.org/ 10.1016/B978-0-12-385071-3.00005-8 [DOI] [PubMed] [Google Scholar]
- 23.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et al.. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26:3213-21; PMID:18591556; http://dx.doi.org/ 10.1200/JCO.2007.15.8923 [DOI] [PubMed] [Google Scholar]
- 24.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, et al.. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004; 351:781-91; PMID:15317891; http://dx.doi.org/ 10.1056/NEJMoa040766 [DOI] [PubMed] [Google Scholar]
- 25.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008; 14:6302-9; PMID:18829513; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-0872 [DOI] [PubMed] [Google Scholar]
- 26.Groot Koerkamp B, Rahbari NN, Buchler MW, Koch M, Weitz J. Circulating Tumor Cells and Prognosis of Patients with Resectable Colorectal Liver Metastases or Widespread Metastatic Colorectal Cancer: A Meta-Analysis. Ann Surg Oncol 2013. Jul; 20(7):2156-65; PMID:23456317; http://dx.doi.org/ 10.1245/s10434-013-2907-8 [DOI] [PubMed] [Google Scholar]
- 27.de Wit S, van Dalum G, Lenferink AT, Tibbe AG, Hiltermann TJ, Groen HJ, van Rijn CJ, Terstappen LW. The detection of EpCAM(+) and EpCAM(-) circulating tumor cells. Sci Rep 2015; 5:12270; PMID:26184843; http://dx.doi.org/ 10.1038/srep12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009; 9:265-73; PMID:19262571; http://dx.doi.org/ 10.1038/nrc2620 [DOI] [PubMed] [Google Scholar]
- 29.Lecharpentier A, Vielh P, Perez-Moreno P, Planchard D, Soria JC, Farace F. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br J Cancer 2011; 105:1338-41; PMID:21970878; http://dx.doi.org/ 10.1038/bjc.2011.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, et al.. EMT and dissemination precede pancreatic tumor formation. Cell; 148:349-61; PMID:22265420; http://dx.doi.org/ 10.1016/j.cell.2011.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikolajczyk SD, Millar LS, Tsinberg P, Coutts SM, Zomorrodi M, Pham T, Bischoff FZ, Pircher TJ. Detection of EpCAM-Negative and Cytokeratin-Negative Circulating Tumor Cells in Peripheral Blood. J Oncol 2011; 2011:252361; PMID:21577258; http://dx.doi.org/ 10.1155/2011/252361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konigsberg R, Gneist M, Jahn-Kuch D, Pfeiler G, Hager G, Hudec M, Dittrich C, Zeillinger R. Circulating tumor cells in metastatic colorectal cancer: efficacy and feasibility of different enrichment methods. Cancer Lett 2010; 293:117-23; PMID:20167419; http://dx.doi.org/ 10.1016/j.canlet.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 33.Zheng S, Lin HK, Liu J-Q, Balic M, Datar R, Cote RJ, Tai Y-C. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J Chromatogr A 2007; 1162:154-61; PMID:17561026; http://dx.doi.org/ 10.1016/j.chroma.2007.05.064 [DOI] [PubMed] [Google Scholar]
- 34.Lin HK, Zheng S, Williams AJ, Balic M, Groshen S, Scher HI, Fleisher M, Stadler W, Datar RH, Tai YC, Cote RJ. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin Cancer Res 2010; 16:5011-8; PMID:20876796; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleischer RL, Price PB, Symes EM. Novel Filter for Biological Materials. Science 1964; 143:249-50; PMID:17753151; http://dx.doi.org/ 10.1126/science.143.3603.249 [DOI] [PubMed] [Google Scholar]
- 36.Seal SH. A sieve for the isolation of cancer cells and other large cells from the blood. Cancer 1964; 17:637-42; PMID:14159810; http://dx.doi.org/ 10.1002/1097-0142(196405)17:5%3c637::AID-CNCR2820170512%3e3.0.CO;2-I [DOI] [PubMed] [Google Scholar]
- 37.Fleischer R, Alter H, Furman S, Price P, Walker R. Particle Track Etching Diverse technological uses range from virus identification to uranium exploration. Science 1972; 178:255-63; PMID:5078248; http://dx.doi.org/ 10.1126/science.178.4058.255 [DOI] [PubMed] [Google Scholar]
- 38.Vona G, Estepa L, Beroud C, Damotte D, Capron F, Nalpas B, Mineur A, Franco D, Lacour B, Pol S, et al.. Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology 2004; 39:792-7; PMID:14999698; http://dx.doi.org/ 10.1002/hep.20091 [DOI] [PubMed] [Google Scholar]
- 39.Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schutze K, Capron F, Franco D, Pazzagli M, Vekemans M, et al.. Isolation by size of epithelial tumor cells - A new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol 2000; 156:57-63; PMID:10623654; http://dx.doi.org/ 10.1016/S0002-9440(10)64706-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clawson GA, Kimchi E, Patrick SD, Xin P, Harouaka R, Zheng S, Berg A, Schell T, Staveley-O'Carroll KF, Neves RI, Mosca PJ, Thiboutot D. Circulating tumor cells in melanoma patients. PloS one 2012; 7:e41052; PMID:22829910; http://dx.doi.org/ 10.1126/science.1244270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandri MT, Zorzino L, Cassatella MC, Bassi F, Luini A, Casadio C, Botteri E, Rotmensz N, Adamoli L, Nole F. Changes in circulating tumor cell detection in patients with localized breast cancer before and after surgery. Ann Surg Oncol 2010; 17:1539-45; PMID:20135356; http://dx.doi.org/ 10.1245/s10434-010-0918-2 [DOI] [PubMed] [Google Scholar]
- 42.Wind J, Tuynman JB, Tibbe AG, Swennenhuis JF, Richel DJ, van Berge Henegouwen MI, Bemelman WA. Circulating tumour cells during laparoscopic and open surgery for primary colonic cancer in portal and peripheral blood. Eur J Surg Oncol 2009; 35:942-50; PMID:19153024; http://dx.doi.org/ 10.1016/j.ejso.2008.12.003 [DOI] [PubMed] [Google Scholar]
- 43.Deneve E, Riethdorf S, Ramos J, Nocca D, Coffy A, Daures JP, Maudelonde T, Fabre JM, Pantel K, Alix-Panabieres C. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin Chem 2013; 59:1384-92; PMID:23695297; http://dx.doi.org/ 10.1373/clinchem.2013.202846 [DOI] [PubMed] [Google Scholar]
- 44.Jiao LR, Apostolopoulos C, Jacob J, Szydlo R, Johnson N, Tsim N, Habib NA, Coombes RC, Stebbing J. Unique localization of circulating tumor cells in patients with hepatic metastases. J Clin Oncol 2009; 27:6160-5; PMID:19884529; http://dx.doi.org/ 10.1200/JCO.2009.24.5837 [DOI] [PubMed] [Google Scholar]
- 45.Papavasiliou P, Fisher T, Kuhn J, Nemunaitis J, Lamont J. Circulating tumor cells in patients undergoing surgery for hepatic metastases from colorectal cancer. Proc (Bayl Univ Med Cent) 2010; 23:11-4; PMID:20157496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahbari NN, Bork U, Kircher A, Nimitz T, Scholch S, Kahlert C, Schmidt T, Steinert G, Ulrich AB, Reissfelder C, et al.. Compartmental Differences of Circulating Tumor Cells in Colorectal Cancer. Ann Surg Oncol 2012; 19:2195-202; PMID:22230943; http://dx.doi.org/ 10.1245/s10434-011-2178-1 [DOI] [PubMed] [Google Scholar]
- 47.Reddy RM, Murlidhar V, Zhao L, Grabauskiene S, Zhang Z, Ramnath N, Lin J, Chang AC, Carrott P, Lynch W, et al.. Pulmonary venous blood sampling significantly increases the yield of circulating tumor cells in early-stage lung cancer. J Thorac Cardiovasc Surg 2015; 151:852-8; PMID:26614417; http://dx.doi.org/ 10.1016/j.jtcvs.2015.09.126 [DOI] [PubMed] [Google Scholar]
- 48.Hashimoto M, Tanaka F, Yoneda K, Takuwa T, Matsumoto S, Okumura Y, Kondo N, Tsubota N, Tsujimura T, Tabata C, et al.. Significant increase in circulating tumour cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interact Cardiovasc Thorac Surg 2014; 18:775-83; PMID:24618055; http://dx.doi.org/ 10.1093/icvts/ivu048 [DOI] [PubMed] [Google Scholar]
- 49.Huang HB, Ge MJ. The effects of different surgical approaches on the perioperative level of circulating tumor cells in patients with non-small cell lung Cancer. Thorac Cardiovasc Surg 2015; PMID:26030121 [DOI] [PubMed] [Google Scholar]
- 50.Fang ZT, Zhang W, Wang GZ, Zhou B, Yang GW, Qu XD, Liu R, Qian S, Zhu L, Liu LX, et al.. Circulating tumor cells in the central and peripheral venous compartment - assessing hematogenous dissemination after transarterial chemoembolization of hepatocellular carcinoma. Onco Targets Ther 2014; 7:1311-8; PMID:25071374; http://dx.doi.org/ 10.2147/OTT.S62605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Camara O, Kavallaris A, Noschel H, Rengsberger M, Jorke C, Pachmann K. Seeding of epithelial cells into circulation during surgery for breast cancer: the fate of malignant and benign mobilized cells. World J Surg Oncol 2006; 4:67; PMID:17002789; http://dx.doi.org/ 10.1186/1477-7819-4-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Camara O, Kavallaris A, Noschel H, Rengsberger M, Jorke C, Pachmann K. Seeding of epithelial cells into circulation during surgery for breast cancer: the fate of malignant and benign mobilized cells. World J Surg Oncol 2006; 4:67-75; PMID:17002789; http://dx.doi.org/ 10.1186/1477-7819-4-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sawabata N, Okumura M, Utsumi T, Inoue M, Shiono H, Minami M, Nishida T, Sawa Y. Circulating tumor cells in peripheral blood caused by surgical manipulation of non-small-cell lung cancer: pilot study using an immunocytology method. Gen Thorac Cardiovasc Surg 2007; 55:189-92; PMID:17554991; http://dx.doi.org/ 10.1007/s11748-007-0101-2 [DOI] [PubMed] [Google Scholar]
- 54.Ge MJ, Shi D, Wu OC, Wang M, Li LB. Observation of circulating tumour cells in patients with non-small cell lung cancer by real-time fluorescent quantitative reverse transcriptase-polymerase chain reaction in perioperative period. J Cancer Res Clin Oncol 2006; 132:248-56; PMID:16320073; http://dx.doi.org/ 10.1007/s00432-005-0059-3 [DOI] [PubMed] [Google Scholar]
- 55.Dong Q, Huang J, Zhou Y, Li L, Bao G, Feng J, Sha H. Hematogenous dissemination of lung cancer cells during surgery: quantitative deetection by flow cytometry and prognostic significance. Lung Cancer 2002; 37:293-301; PMID:12234699; http://dx.doi.org/ 10.1016/S0169-5002(02)00102-2 [DOI] [PubMed] [Google Scholar]
- 56.Engilbertsson H, Aaltonen KE, Bjornsson S, Kristmundsson T, Patschan O, Ryden L, Gudjonsson S. TURBT can cause seeding of cancer cells into the bloodstream. J Urol 2015. Jan; 193(1):53-7; PMID:24996129; http://dx.doi.org/ 10.1016/j.juro.2014.06.083 [DOI] [PubMed] [Google Scholar]
- 57.Broll R, Lembcke K, Stock C, Zingler M, Duchrow M, Schimmelpenning H, Strik M, Muller G, Kujath P, Bruch HP. Tumor cell dissemination in bone marrow and peritoneal cavity. An immunocytochemical study of patients with stomach or colorectal carcinoma (German). Langenbecks Arch Chir 1996; 381:51-8; PMID:8717176; http://dx.doi.org/ 10.1007/BF00184256 [DOI] [PubMed] [Google Scholar]
- 58.Guller U, Zajac P, Schnider A, Bosch B, Vorburger S, Zuber M, Spagnoli GC, Oertli D, Maurer R, Metzger U, et al.. Disseminated single tumor cells as detected by real-time quantitative plymerase chain reaction represent a prognostic factor in patients undergoing surgery for colorectal cancer. Ann Surg 2002; 236:768-75; PMID:12454515; http://dx.doi.org/ 10.1097/00000658-200212000-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Z, Jiang M, Zhao J, Ju H. Circulating tumor cells in perioperative esophageal cancer patients: quantitative assay system and potential clinical utility. Clin Cancer Res 2007; 13:2992-7; PMID:17505001; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-2072 [DOI] [PubMed] [Google Scholar]
- 60.Sergeant G, Roskams T, van Pelt J, Houtmeyers F, Aerts R, Topal B. Perioperative cancer cell dissemination detected with a real-time RT-PCR assay for EpCAM is not associated with worse prognosis in pancreatic ductal adenocarcinoma. BMC Cancer 2011; 11:47; PMID:21281486; http://dx.doi.org/ 10.1186/1471-2407-11-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ishizuna K, Ota D, Okamoto J, Fukuuchi A, Tanaka R, Fujii A, Mori M, Nishi T. A case of mucinous carcinoma of the breast in which needle tract seeding was diagnosed by preoperative diagnostic imaging. Breast Cancer 2011; 18:324-7; PMID:19701680; http://dx.doi.org/ 10.1007/s12282-009-0151-7 [DOI] [PubMed] [Google Scholar]
- 62.Shyamala K, Girish HC, Murgod S. Risk of tumor cell seeding through biopsy and aspiration cytology. J Int Soc Prev Community Dent 2014; 4:5-11; PMID:24818087; http://dx.doi.org/ 10.4103/2231-0762.129446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marrinucci D, Bethel K, Kolatkar A, Luttgen MS, Malchiodi M, Baehring F, Voigt K, Lazar D, Nieva J, Bazhenova L, et al.. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol 2012; 9:016003; PMID:22306768; http://dx.doi.org/ 10.1088/1478-3975/9/1/016003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stott SL, Hsu C-H, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci U S A 2010; 107:18392-7; PMID:20930119; http://dx.doi.org/ 10.1073/pnas.1012539107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hou HW, Warkiani ME, Khoo BL, Li ZR, Soo RA, Tan DS-W, Lim W-T, Han J, Bhagat AAS, Lim CT. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci Rep 2013; 3:1259; PMID:AMBIGUOUS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM. Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science 2013; 339:580-4; PMID:23372014; http://dx.doi.org/ 10.1126/science.1228522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fidler IJ. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer 1973; 9:223-7; PMID:4787857; http://dx.doi.org/ 10.1016/S0014-2964(73)80022-2 [DOI] [PubMed] [Google Scholar]
- 68.Liotta LA, Kleinerman J, Saldel GM. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res 1976; 36:889-94; PMID:1253177 [PubMed] [Google Scholar]
- 69.Lip GY, Chin BS, Blann AD. Cancer and the prothrombotic state. Lancet Oncol 2002; 3:27-34; PMID:11908507; http://dx.doi.org/ 10.1016/S1470-2045(01)00619-2 [DOI] [PubMed] [Google Scholar]
- 70.De Martino RR, Goodney PP, Spangler EL, Wallaert JB, Corriere MA, Rzucidlo EM, Walsh DB, Stone DH. Variation in thromboembolic complications among patients undergoing commonly performed cancer operations. J Vasc Surg 2012; 55:1035-40 e4; PMID:22409858; http://dx.doi.org/ 10.1016/j.jvs.2011.10.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massague J. Tumor self-seeding by circulating cancer cells. Cell 2009; 139:1315-26; PMID:20064377; http://dx.doi.org/ 10.1016/j.cell.2009.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alix-Panabieres C, Pantel K. Circulating Tumor Cells: Liquid Biopsy of Cancer. Clin Chem 2012; 2008 Aug 15; 14(16):5013-21; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-5125 [DOI] [PubMed] [Google Scholar]
- 73.Alix-Panabieres C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res 2008; 14:5013-21; PMID:18698019; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-5125 [DOI] [PubMed] [Google Scholar]
- 74.Bidard FC, Kirova YM, Vincent-Salomon A, Alran S, de Rycke Y, Sigal-Zafrani B, Sastre-Garau X, Mignot L, Fourquet A, Pierga JY. Disseminated tumor cells and the risk of locoregional recurrence in nonmetastatic breast cancer. Ann Oncol 2009; 20:1836-41; PMID:19556319; http://dx.doi.org/ 10.1093/annonc/mdp200 [DOI] [PubMed] [Google Scholar]
- 75.Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, et al.. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 2005; 353:793-802; PMID:16120859; http://dx.doi.org/ 10.1056/NEJMoa050434 [DOI] [PubMed] [Google Scholar]
- 76.Kaifi JT, Yekebas EF, Schurr P, Obonyo D, Wachowiak R, Busch P, Heinecke A, Pantel K, Izbicki JR. Tumor-cell homing to lymph nodes and bone marrow and CXCR4 expression in esophageal cancer. J Natl Cancer Inst 2005; 97:1840-7; PMID:16368946; http://dx.doi.org/ 10.1093/jnci/dji431 [DOI] [PubMed] [Google Scholar]
- 77.Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, Datar RH, Cote RJ. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res 2006; 12:5615-21; PMID:17020963; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-0169 [DOI] [PubMed] [Google Scholar]
- 78.Gros SJ, Kurschat N, Drenckhan A, Dohrmann T, Forberich E, Effenberger K, Reichelt U, Hoffman RM, Pantel K, Kaifi JT et al., Involvement of CXCR4 chemokine receptor in metastastic HER2-positive esophageal cancer. PloS one 2012; 7:e47287; PMID:23082154; http://dx.doi.org/ 10.1371/journal.pone.0047287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang L, Ridgway LD, Wetzel MD, Ngo J, Yin W, Kumar D, Goodman JC, Groves MD, Marchetti D. The Identification and Characterization of Breast Cancer CTCs Competent for Brain Metastasis. Sci Transl Med 2013; 5:180ra48; PMID:23576814; http://dx.doi.org/ 10.1126/scitranslmed.3005109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mego M, De Giorgi U, Dawood S, Wang X, Valero V, Andreopoulou E, Handy B, Ueno NT, Reuben JM, Cristofanilli M. Characterization of metastatic breast cancer patients with nondetectable circulating tumor cells. Int J Cancer 2011; 129:417-23; PMID:20857493; http://dx.doi.org/ 10.1002/ijc.25690 [DOI] [PubMed] [Google Scholar]
- 81.Harouaka RC, Zhou MD, Yeh YT, Khan WJ, Das A, Liu X, Christ CC, Dicker DT, Baney TS, Kaifi JT et al., Flexible Micro Spring Array Device for High Throughput Enrichment of Viable Circulating Tumor Cells. 2014 Feb; 60(2):323-33; PMID:24132944; http://dx.doi.org/ 10.1373/clinchem.2013.206805 [DOI] [PubMed] [Google Scholar]
- 82.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119:1420-8; PMID:19487818; http://dx.doi.org/ 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, Herold CI, Marcom PK, George DJ, Garcia-Blanco MA. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res 2011; 9:997-1007; PMID:21665936; http://dx.doi.org/ 10.1158/1541-7786.MCR-10-0490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lecharpentier A, Vielh P, Perez-Moreno P, Planchard D, Soria J, Farace F. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br J Cancer 2011; 105:1338-41; PMID:21970878; http://dx.doi.org/ 10.1038/bjc.2011.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaifi JT, Reichelt U, Quaas A, Schurr PG, Wachowiak R, Yekebas EF, Strate T, Schneider C, Pantel K, Schachner M et al., L1 is associated with micrometastatic spread and poor outcome in colorectal cancer. Mod Pathol 2007; 20:1183-90; PMID:17873897; http://dx.doi.org/ 10.1038/modpathol.3800955 [DOI] [PubMed] [Google Scholar]
- 86.Gasch C, Bauernhofer T, Pichler M, Langer-Freitag S, Reeh M, Seifert AM, Mauermann O, Izbicki JR, Pantel K et al., Heterogeneity of Epidermal Growth Factor Receptor Status and Mutations of KRAS/PIK3CA in Circulating Tumor Cells of Patients with Colorectal Cancer. Clin Chem. 2013. Jan; 59(1):252-60; PMID:23136247; http://dx.doi.org/ 10.1373/clinchem.2012.188557 [DOI] [PubMed] [Google Scholar]
- 87.Riethdorf S, Muller V, Zhang L, Rau T, Loibl S, Komor M, Roller M, Huober J, Fehm T, Schrader I et al., Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res 2010; 16:2634-45; PMID:20406831; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-2042 [DOI] [PubMed] [Google Scholar]
- 88.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer 2008; 8:329-40; PMID:18404148; http://dx.doi.org/ 10.1038/nrc2375 [DOI] [PubMed] [Google Scholar]
- 89.Park Y, Kitahara T, Urita T, Yoshida Y, Kato R. Expected clinical applications of circulating tumor cells in breast cancer. World J Clin Oncol 2011; 2:303-10; PMID:21876851; http://dx.doi.org/ 10.5306/wjco.v2.i8.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cayrefourcq L, Mazard T, Joosse S, Solassol J, Ramos J, Assenat E, Schumacher U, Costes V, Maudelonde T, Pantel K et al., Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res 2015; 75:892-901; PMID:25592149; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-2613 [DOI] [PubMed] [Google Scholar]
- 91.Aichel O. Ueber Zellverschmelzung mit qualitativ abnormer Chromosomenverteilung als Ursache der Geschwulstbildung. Heft XIII herausgegebenen von Wilhelm Roux (Verlag von Wilhelm Engelmann, Leipzig) 1911; XIII:92-111. [Google Scholar]
- 92.Clawson GA. Cancer. Fusion for moving. Science 2013; 342:699-700; PMID:24202164; http://dx.doi.org/ 10.1126/science.1244270 [DOI] [PubMed] [Google Scholar]
- 93.Steinert G, Scholch S, Niemietz T, Iwata N, Garcia SA, Behrens B, Voigt A, Kloor M, Benner A, Bork U et al., Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res 2014; 74:1694-704; PMID:24599131; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-1885 [DOI] [PubMed] [Google Scholar]
- 94.Raynor MP, Stephenson SA, Pittman KB, Walsh DC, Henderson MA, Dobrovic A. Identification of circulating tumour cells in early stage breast cancer patients using multi marker immunobead RT-PCR. J Hematol Oncol 2009; 2:24; PMID:19500345; http://dx.doi.org/ 10.1186/1756-8722-2-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maltoni R, Fici P, Amadori D, Gallerani G, Cocchi C, Zoli M, Rocca A, Cecconetto L, Folli S, Scarpi E et al., Circulating tumor cells in early breast cancer: A connection with vascular invasion. Cancer Lett 2015; 367:43-8; PMID:26184997; http://dx.doi.org/ 10.1016/j.canlet.2015.06.020 [DOI] [PubMed] [Google Scholar]
- 96.Wu P, Tang RN, Zou JH, Wang FC. The prognostic role of disseminated tumor cells detected in peripheral blood and bone marrow of colorectal cancer. Hepatogastroenterology 2012; 59:2164-7; PMID:23178553; http://dx.doi.org/ 10.5754/hge12539 [DOI] [PubMed] [Google Scholar]
- 97.Soeth E, Vogel I, Roder C, Juhl H, Marxsen J, Kruger U, Henne-Bruns D, Kremer B, Kalthoff H. Comparative analysis of bone marrow and venous blood isolates from gastrointestinal cancer patients for the detection of disseminated tumor cells using reverse transcription PCR. Cancer Res 1997; 57:3106-10; PMID:9242433 [PubMed] [Google Scholar]
- 98.Lindemann F, Schlimok G, Dirschedl P, Witte J, Riethmuller G. Prognostic significance of micrometastatic tumour cells in bone marrow of colorectal cancer patients. Lancet 1992; 340:685-9; PMID:1381801; http://dx.doi.org/ 10.1016/0140-6736(92)92230-D [DOI] [PubMed] [Google Scholar]
- 99.Reeh M, Effenberger KE, Koenig AM, Riethdorf S, Eichstadt D, Vettorazzi E, Uzunoglu FG, Vashist YK, Izbicki JR, Pantel K et al., Circulating Tumor Cells as a Biomarker for Preoperative Prognostic Staging in Patients With Esophageal Cancer. Ann Surg 2015; 261:1124-30; PMID:25607767; http://dx.doi.org/ 10.1097/SLA.0000000000001130 [DOI] [PubMed] [Google Scholar]
- 100.Thorban S, Roder JD, Nekarda H, Funk A, Siewert JR, Pantel K. Immunocytochemical detection of disseminated tumor cells in the bone marrow of patients with esophageal carcinoma. J Natl Cancer Inst 1996; 88:1222-7; PMID:8780632; http://dx.doi.org/ 10.1093/jnci/88.17.1222 [DOI] [PubMed] [Google Scholar]
- 101.Kolostova K, Matkowski R, Gurlich R, Grabowski K, Soter K, Lischke R, Schutzner J, Bobek V. Detection and cultivation of circulating tumor cells in gastric cancer. Cytotechnology 2015; PMID:25862542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li M, Zhang B, Zhang Z, Liu X, Qi X, Zhao J, Jiang Y, Zhai H, Ji Y, Luo D. Stem cell-like circulating tumor cells indicate poor prognosis in gastric cancer. Biomed Res Int 2014; 2014:981261; PMID:24963492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Allgayer H, Heiss MM, Riesenberg R, Babic R, Jauch KW, Schildberg FW. Immunocytochemical phenotyping of disseminated tumor cells in bone marrow by uPA receptor and CK18: investigation of sensitivity and specificity of an immunogold/alkaline phosphatase double staining protocol. J Histochem Cytochem 1997; 45:203-12; PMID:9016310; http://dx.doi.org/ 10.1177/002215549704500206 [DOI] [PubMed] [Google Scholar]
- 104.Heiss MM, Simon EH, Beyer BC, Gruetzner KU, Tarabichi A, Babic R, Schildberg FW, Allgayer H. Minimal residual disease in gastric cancer: evidence of an independent prognostic relevance of urokinase receptor expression by disseminated tumor cells in the bone marrow. J Clin Oncol 2002; 20:2005-16; PMID:11956259; http://dx.doi.org/ 10.1200/JCO.2002.08.003 [DOI] [PubMed] [Google Scholar]
- 105.Inhestern J, Oertel K, Stemmann V, Schmalenberg H, Dietz A, Rotter N, Veit J, Gorner M, Sudhoff H, Junghanss C et al., Prognostic Role of Circulating Tumor Cells during Induction Chemotherapy Followed by Curative Surgery Combined with Postoperative Radiotherapy in Patients with Locally Advanced Oral and Oropharyngeal Squamous Cell Cancer. PloS one 2015; 10:e0132901; PMID:26186556; http://dx.doi.org/ 10.1371/journal.pone.0132901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hristozova T, Konschak R, Stromberger C, Fusi A, Liu Z, Weichert W, Stenzinger A, Budach V, Keilholz U, Tinhofer I. The presence of circulating tumor cells (CTCs) correlates with lymph node metastasis in nonresectable squamous cell carcinoma of the head and neck region (SCCHN). Ann Oncol 2011; 22:1878-85; PMID:21525401; http://dx.doi.org/ 10.1093/annonc/mdr130 [DOI] [PubMed] [Google Scholar]
- 107.Partridge M, Brakenhoff R, Phillips E, Ali K, Francis R, Hooper R, Lavery K, Brown A, Langdon J. Detection of rare disseminated tumor cells identifies head and neck cancer patients at risk of treatment failure. Clin Cancer Res 2003; 9:5287-94; PMID:14614011 [PubMed] [Google Scholar]
- 108.Partridge M, Phillips E, Francis R, Li SR. Immunomagnetic separation for enrichment and sensitive detection of disseminated tumour cells in patients with head and neck SCC. J Pathol 1999; 189:368-77; PMID:10547599; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199911)189:3%3c368::AID-PATH441%3e3.0.CO;2-2 [DOI] [PubMed] [Google Scholar]
- 109.Tanaka F, Yoneda K, Kondo N, Hashimoto M, Takuwa T, Matsumoto S, Okumura Y, Rahman S, Tsubota N, Tsujimura T et al., Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res 2009; 15:6980-6; PMID:19887487; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-1095 [DOI] [PubMed] [Google Scholar]
- 110.Hofman V, Bonnetaud C, Ilie MI, Vielh P, Vignaud JM, Flejou JF, Lantuejoul S, Piaton E, Mourad N, Butori C, et al.. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res 2010; 17:827-35; PMID:21098695; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-0445 [DOI] [PubMed] [Google Scholar]
- 111.Pantel K, Izbicki J, Passlick B, Angstwurm M, Haussinger K, Thetter O, Riethmuller G. Frequency and prognostic significance of isolated tumour cells in bone marrow of patients with non-small-cell lung cancer without overt metastases. Lancet 1996; 347:649-53; PMID:8596379; http://dx.doi.org/ 10.1016/S0140-6736(96)91203-9 [DOI] [PubMed] [Google Scholar]
- 112.Cote RJ, Beattie EJ, Chaiwun B, Shi SR, Harvey J, Chen SC, Sherrod AE, Groshen S, Taylor CR. Detection of occult bone marrow micrometastases in patients with operable lung carcinoma. Ann Surg 1995; 222:415-23; discussion 23-5; PMID:7574923; http://dx.doi.org/ 10.1097/00000658-199522240-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Passlick B, Kubuschok B, Izbicki JR, Thetter O, Pantel K. Isolated tumor cells in bone marrow predict reduced survival in node-negative non-small cell lung cancer. Ann Thorac Surg 1999; 68:2053-8; PMID:10616976; http://dx.doi.org/ 10.1016/S0003-4975(99)01125-X [DOI] [PubMed] [Google Scholar]
- 114.Earl J, Garcia-Nieto S, Martinez-Avila JC, Montans J, Sanjuanbenito A, Rodriguez-Garrote M, Lisa E, Mendia E, Lobo E, Malats N et al., Circulating tumor cells (Ctc) and kras mutant circulating free Dna (cfdna) detection in peripheral blood as biomarkers in patients diagnosed with exocrine pancreatic cancer. BMC Cancer 2015; 15:797; PMID:26498594; http://dx.doi.org/ 10.1186/s12885-015-1779-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cauley CE, Pitman MB, Zhou J, Perkins J, Kuleman B, Liss AS, Fernandez-Del Castillo C, Warshaw AL, Lillemoe KD, Thayer SP. Circulating Epithelial Cells in Patients with Pancreatic Lesions: Clinical and Pathologic Findings. J Am Coll Surg 2015; 221:699-707; PMID:26209458; http://dx.doi.org/ 10.1016/j.jamcollsurg.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Soeth E, Grigoleit U, Moellmann B, Roder C, Schniewind B, Kremer B, Kalthoff H, Vogel I. Detection of tumor cell dissemination in pancreatic ductal carcinoma patients by CK 20 RT-PCR indicates poor survival. J Cancer Res Clin Oncol 2005; 131:669-76; PMID:16136352; http://dx.doi.org/ 10.1007/s00432-005-0008-1 [DOI] [PubMed] [Google Scholar]
- 117.Z'Graggen K, Centeno BA, Fernandez-del Castillo C, Jimenez RE, Werner J, Warshaw AL. Biological implications of tumor cells in blood and bone marrow of pancreatic cancer patients. Surgery 2001; 129:537-46; PMID:11331445; http://dx.doi.org/ 10.1067/msy.2001.113819 [DOI] [PubMed] [Google Scholar]
- 118.Khurana KK, Grane R, Borden EC, Klein EA. Prevalence of circulating tumor cells in localized prostate cancer. Curr Urol 2014; 7:65-9; PMID:24917761; http://dx.doi.org/ 10.1159/000356251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shao C, Liao CP, Hu P, Chu CY, Zhang L, Bui MH, Ng CS, Josephson DY, Knudsen B, Tighiouart M et al., Detection of live circulating tumor cells by a class of near-infrared heptamethine carbocyanine dyes in patients with localized and metastatic prostate cancer. PloS One 2014; 9:e88967; PMID:24551200; http://dx.doi.org/ 10.1371/journal.pone.0088967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pantel K, Enzmann T, Kollermann J, Caprano J, Riethmuller G, Kollermann MW. Immunocytochemical monitoring of micrometastatic disease: reduction of prostate cancer cells in bone marrow by androgen deprivation. Int J Cancer 1997; 71:521-5; PMID:9178803; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19970516)71:4%3c521::AID-IJC4%3e3.0.CO;2-W [DOI] [PubMed] [Google Scholar]
- 121.Kollermann J, Heseding B, Helpap B, Kollermann MW, Pantel K. Comparative immunocytochemical assessment of isolated carcinoma cells in lymph nodes and bone marrow of patients with clinically localized prostate cancer. Int J Cancer 1999; 84:145-9; PMID:10096246; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19990420)84:2%3c145::AID-IJC9%3e3.0.CO;2-5 [DOI] [PubMed] [Google Scholar]
- 122.Pantel K, Aignherr C, Kollermann J, Caprano J, Riethmuller G, Kollermann MW. Immunocytochemical detection of isolated tumour cells in bone marrow of patients with untreated stage C prostatic cancer. Eur J Cancer 1995; 31A:1627-32; PMID:7488413; http://dx.doi.org/ 10.1016/0959-8049(95)00290-Y [DOI] [PubMed] [Google Scholar]


