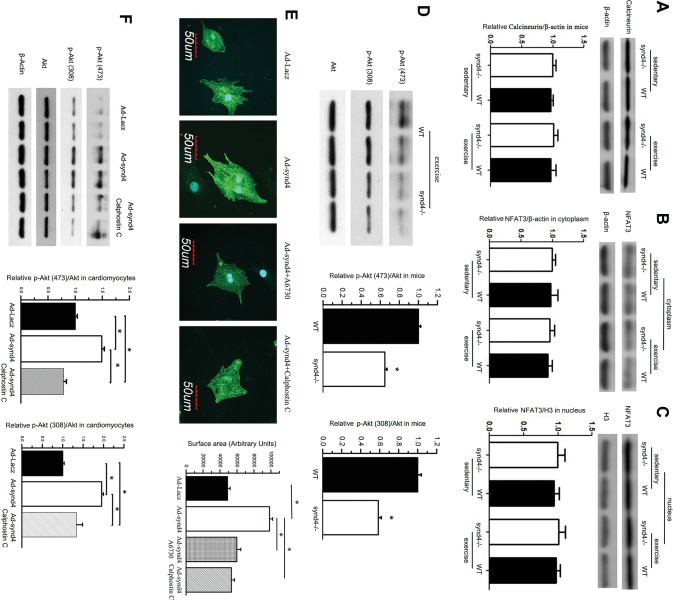
Figure 5.
Synd4 induced cardiomyocytes hypertrophy via Akt phosphorylation. (A–C) Western blot showed no changes of (A) calcineurin and (B and C) its downstream targets, NFAT3 in myocardium between WT and synd4–/– mice, as well as between sedentary and exercise mice. Data are shown as a ratio to β-actin or histone H3 and are expressed as a fold-increase when compared with sedentary synd4–/– mice. N = 6, ANOVA; (D) Western blot showed decreased S473 and T308 Akt phosphorylation in synd4–/– mice after exercise. Data are shown as a ratio to total Akt and are expressed as a fold-increase when compared with WT mice. *P < 0.05, synd4–/– mice versus WT mice, N = 6, ANOVA; Ad-synd4 was used to activate synd4 in cultured cardiomyocyte. (E) Immunofluorescence assay showed synd4 overexpression could induce cardiomyocyte enlargement. A6730 (Akt inhibitor, 5 μmol/L) and calphostin C (PKC inhibitor, 100 nmol/L) could attenuate synd4 depended cardiomyocyte enlargement. *P < 0.05, Ad-Lacz versus Ad-synd4, Ad-synd4 + A6730 versus Ad-synd4, Ad-synd4 + calphostin C versus Ad-synd4; N = 3 experiments, ANOVA; (F) Western blot showed synd4 overexpression could increase Akt phosphorylation in S473 and T308. Calphostin C could blunt synd4-induced Akt phosphorylation. Data are shown as a ratio to total Akt and are expressed as a fold-increase when compared with Ad-Lacz groups. *P < 0.05, Ad-Lacz versus Ad-synd4, Ad-synd4 + calphostin C versus Ad-synd4; N = 3 experiments, ANOVA.