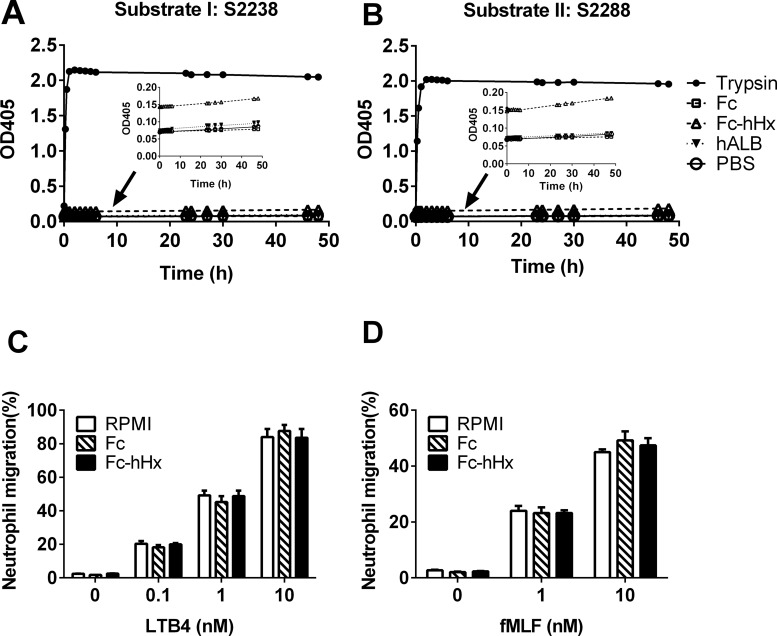
Figure 5.
Recombinant Fc-hHx has minimal protease activity and no inhibition on the chemotaxis of human neutrophils induced by fMLF and LTB4. Enzymatic activity of recombinant Fc-human Hx (Fc-hHx) was tested by using chromogenic substrates substrate I: S2238 (A) and II: S2288 (B). Fc-hHx and the tag Fc at the concentration of 6.7 μmol/L, positive control trypsin (0.4 μmol/L), negative control human albumin (hALB) and PBS were mixed with the substrate at desired concentrations and incubated at 37°C for different times. The absorbance at 405 nm was measured by spectrophotometry. The small inserts are the sections with small scale and without trypsin control to show the very low increase of the absorbance by Fc-hHx (C) and (D): Fc or Fc-hHx at 100 μg/mL (1.67 μmol/L) were also used to pretreat human neutrophils with control (RPMI 1640 containing 2% FBS) for 1 h. Then 1–2.5 × 105 cells/30 μL cells were applied to the top of the transwell with the lower microplate filled with LTB4 (0.1 nmol/L, 1 nmol/L and 10 nmol/L) (C) or fMLF (1 nmol/L and 10 nmol/L) (D) and incubated for 1 h. Neutrophils migrated to the bottom microplate wells were stained with 0.4% trypan blue and live cells were counted. The percentage of cells migrated over the total cells uploaded to the top of the transwell was calculated. The results represent the mean ± SE and are representative of three independent experiments.