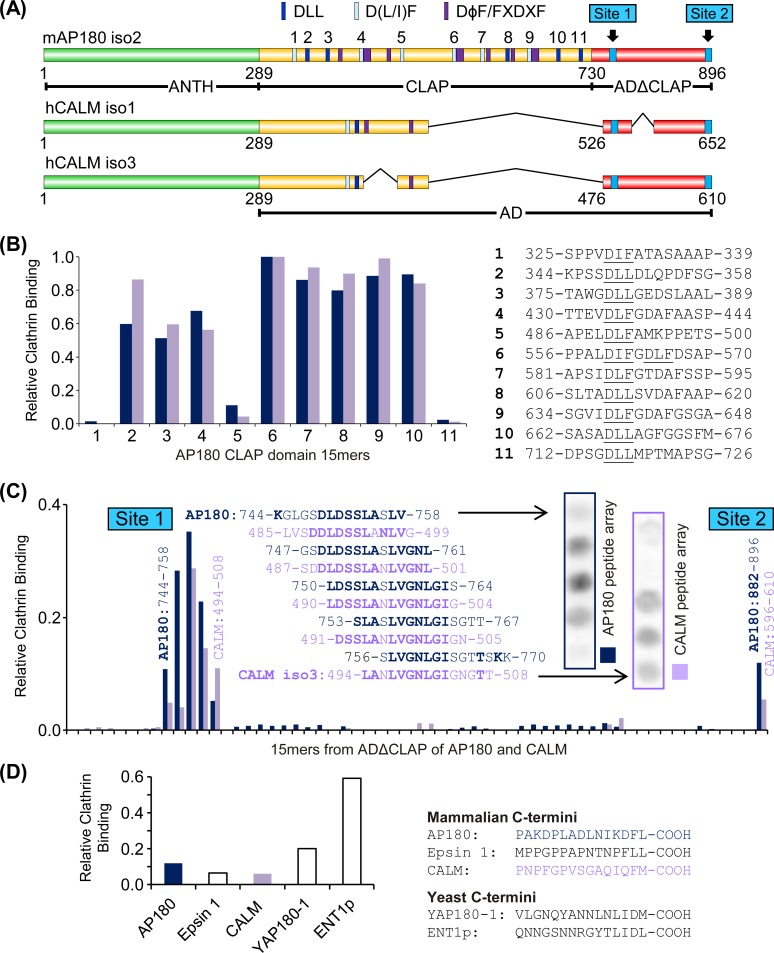
Fig 1. Binding of clathrin to 15mer peptides from the AP180 CLAP domain and AP180 and CALM ADΔCLAP domains.
(A) Diagram indicating AP180 and CALM domains, the location of existing clathrin heavy chain (CHC) and adapter protein complex 2 (AP2) binding motifs [7] and the clathrin binding sites identified in this work. Note CALM isoform 3 was used to obtain results in this figure and Fig 2. CALM isoform 1 was used in subsequent figures. (B) The relative amount of purified clathrin bound to AP180 15mers containing known CBMs in an overlay assay. Numbers correspond to numbered binding motifs in (A). The dark blue columns and purple columns correspond to the overlay experiment with the AP180 and CALM ADΔCLAP 15mers, respectively. The intensity for each peptide was divided by the intensity of clathrin binding to peptide 6. (C) The amount of clathrin binding to overlapping 15mer peptides from the ADΔCLAP of AP180 isoform 2 and CALM isoform 3 (see Figure A in S1 File for full list of aligned peptides), relative to peptide 6 in (B). Same colour scheme as (B). Sequences and spots for Site 1 are shown. Bold residues in sequence alignment indicate identity between AP180 and CALM. The respective C-termini from AP180 and CALM are both referred to as Site 2. (D) Clathrin binding for AP180 and CALM Site 2 15mers compared to 15mer peptides from the C-termini of other clathrin adaptors (normalized to peptide 6 in (B), n = 1).