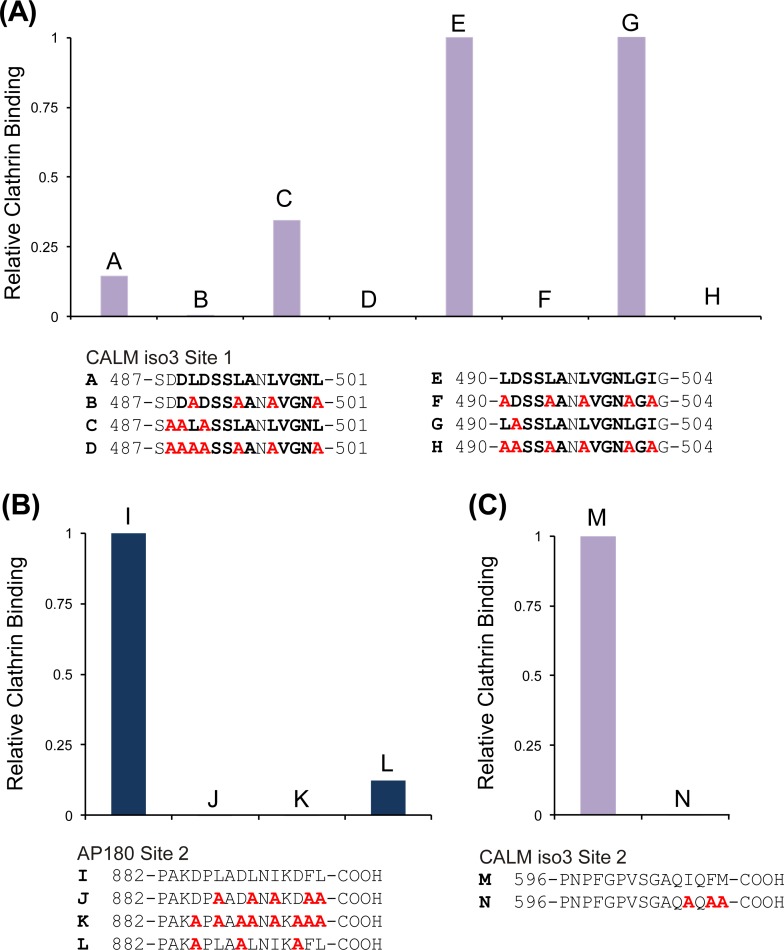
Fig 2. Substitution of amino acid residues in peptides from Site 1 and Site 2 to identify those important for clathrin binding.
Hydrophobic amino acids (Leu, Ile, Met, and Phe), Asp residues and conserved residues in the extreme C-terminus were substituted with Ala (red residues) in peptide array overlay assays with purified clathrin. Bold residues indicate conserved amino acids. (A) CALM Site 1 substitutions and their effect on clathrin binding to 15mers (n = 1). The intensity for each 15mer has been divided by the intensity of clathrin binding to 15mer E (B) AP180 Site 2 substitutions and their effect on clathrin binding to 15mers (n = 1). The intensity for each 15mer has been divided by the intensity of clathrin binding to 15mer I (C) CALM Site 2 substitutions and their effect on clathrin binding to 15mers (n = 1). The intensity for each 15mer was divided by the intensity of clathrin binding to 15mer M.