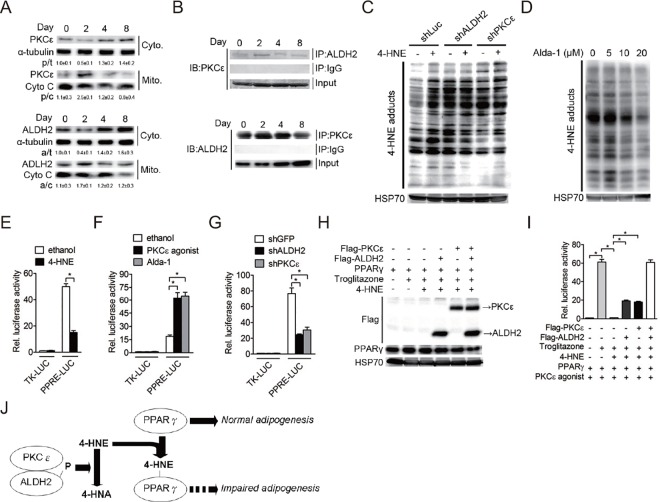
Fig 4. PKCε-ALDH2 interaction involves in regulation of PPARγ activity.
A, Cytoplasmic and mitochondrial distribution of PKCε and ALDH2 protein throughout the differentiation process (n = 3 independent experiments). The results shown are representative of an individual experiment. The ratio of intensity of bands corresponding to target protein per loading control was analyzed by densitometer software. The numbers indicate the means ± SE. B, PKCε coimmunoprecipitates with ALDH2 during adipocyte differentiation. Cell extracts harvested from differentiating adipocytes were immunoprecipitated (IP) with either IgG, ALDH2 or PKCε antibody. The immunoblots (IB) were probed for either PKCε or ALDH2 antibody. Input is shown in the lower panels (n = 4 independent experiments). The results shown are representative of an individual experiment. C, shRNA control, ALDH2-knockdown and PKCε-knockdown preadipocytes were treated with or without 10 μM 4-hydroxynonenal (4-HNE) for 24 hours. Total cell extracts were harvested and analyzed by immunoblotting with anti-4-HNE antibody (n = 4 independent experiments). The results shown are representative of an individual experiment. D, Effect of Alda-1 on 4-HNE formation. 3T3-L1 preadipocytes were maintained in induction medium with various dose of Alda-1 for 2 days. After 8 day of adipogenic stimulation, total cell extracts were harvested and analyzed by immunoblotting with anti-4-HNE antibody (n = 4 independent experiments). The results shown are representative of an individual experiment. E, 4-HNE attenuates PPRE-driven luciferase activity in differentiating 3T3-L1 cells. Differentiating 3T3-L1 cells were transfected with reporter vectors (TK-LUC and PPRE-LUC) for 24 hour and then incubated cells in induction medium with or without treatments (10 μM 4-HNE) for 24 hour. The activity of firefly luciferase was determined and normalized to the activity of renilla luciferase. Data are shown as mean ± SE from 4 independent experiments. * P < 0.05 versus ethanol. F, PKCε agonist and Alda-1 trigger PPRE-driven luciferase activity in differentiating 3T3-L1 cells. Differentiating 3T3-L1 cells were transfected with reporter vectors (TK-LUC and PPRE-LUC) for 24 hour and then incubated cells in induction medium with or without treatments (1 μM PKCε agonist and 10 μM Alda-1) for 2 days. The activity of firefly luciferase was determined and normalized to the activity of renilla luciferase. Data are shown as mean ± SE from 4 independent experiments. * P < 0.05 versus ethanol. G, Silencing of ALDH2 or PKCε reduces PPRE-driven luciferase activity in differentiating 3T3-L1 cells. Differentiating shGFP, shALDH2 and shPKCε cells were transfected with reporter vectors (TK-LUC and PPRE-LUC) for 24 hour. The activity of firefly luciferase was determined and normalized to the activity of renilla luciferase. Data are shown as mean ± SE from 4 independent experiments. * P < 0.05 versus shGFP. H, PKCε-ALDH2 pathway potentiates PPARγ transcriptional activity. 293 cells were transiently transfected with expression vectors (Flag-ALDH2, Flag-PKCε and CMX-GAL4-PPARγ) and UASG×4-TK-LUC reporter plasmid for 24 hour and then incubated cells in growth medium with 1 μM PKCε agonist, 1 μM troglitazone and 10 μM 4-HNE for 2 days (n = 3 independent experiments). The results shown are representative of an individual experiment. I, The activity of firefly luciferase was determined and normalized to the activity of renilla luciferase. Data are shown as mean ± SE from 4 independent experiments. * P < 0.05 versus control cells. J, Proposed model for the mechanism by which PKCε-ALDH2 interaction involves in regulation of PPARγ activity by 4-HNE metabolism.