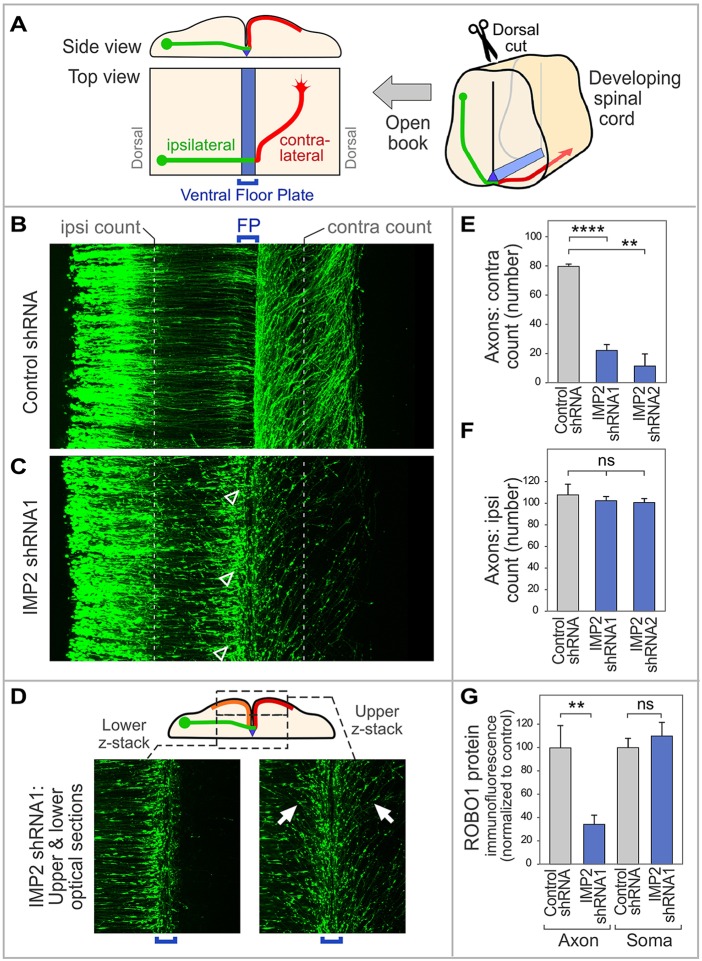
Fig. 4.
IMP2 knockdown causes defects in commissural axon pathfinding in vivo. (A) Diagram of spinal cord open-book preparation. The developing spinal cord is cut open along the dorsal midline, then mounted ventricular face downwards. A typical commissural axon ipsilateral segment (green), contralateral segment (red) and the floor plate (blue) are shown. (B-F) IMP2 or control shRNAs were introduced by in ovo electroporation into one side of the chick embryo spinal cord, with a Math1-GFP construct to trace commissural axons. Spinal cord was dissected 64 h later and imaged as an open-book. (B) Commissural axons in a confocal z-stack of open-book, viewed from the top. Electroporated cell bodies are visible on the left, and axons grow toward the right. (C) After IMP2 knockdown, in contrast to the control many growth cones are seen to have stalled at or near the ipsilateral side of the floor plate, showing an enlarged fusiform morphology typical of stalled growth cones (arrowheads). Correspondingly, a reduced number of axons was seen on the contralateral side, where they appeared to follow roughly normal trajectories without obvious signs of degeneration. (D) The lower confocal z-stack shows axon segments approaching the floor plate (green in diagram). The upper z-stack shows axon trajectories diverging away from the midline on both sides (contralateral, red in diagram; and ipsilateral, orange in diagram; examples of the approximately mirror image trajectories are arrowed). (E,F) Quantitation of ipsilateral and contralateral axon numbers, counted at the positions indicated by the white dashed lines in B and C. IMP2 knockdown did not significantly affect the number of axons emerging from the dorsal commissural neuron cell bodies and growing toward the floor plate, but strongly reduced the percentage of axons on the contralateral side. Each replicate was a separate embryo. (G) Effect of IMP2 RNAi on Robo1 expression in spinal commissural neurons. IMP2 or control shRNAs were introduced by in ovo electroporation into chick embryo spinal cord, with a Math1-GFP construct to identify transfected neurons; 64 h later, commissural neurons were dissociated and cultured for analysis of Robo1 immunofluorescence in axons and cell bodies. Immunolabeling was performed on permeabilized cells to detect both intracellular and cell surface Robo1. IMP2 shRNA reduced Robo1 expression in the axon, but not in the soma (see also Fig. S4B). Each replicate in this experiment was a different neuron (n=16 for control and n=28 for IMP2 shRNA), and the experiment was repeated three times to ensure reproducibility. Comparisons used Student's unpaired two-tailed t-test. Error bars show s.e.m. **P<0.01, ****P<0.0001; ns, not significant.