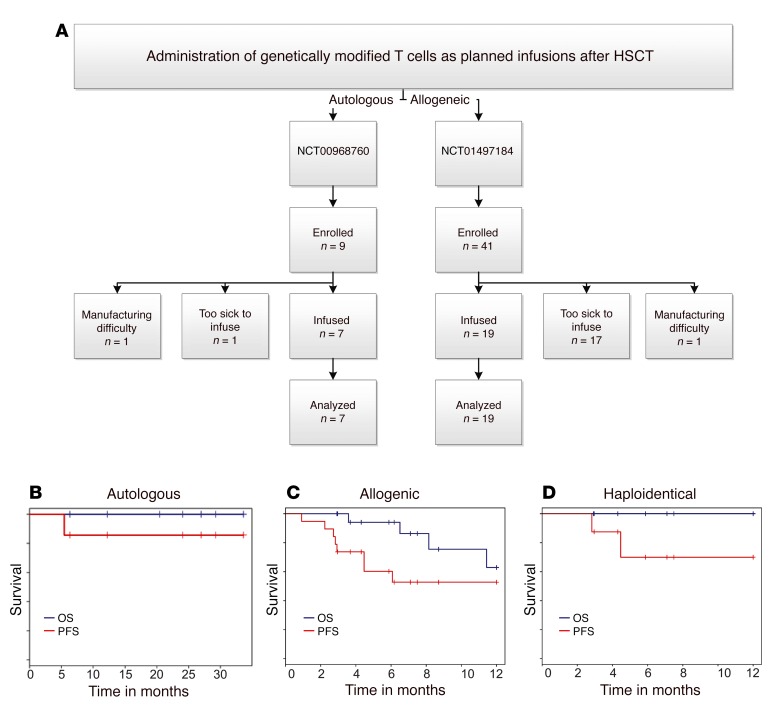
Figure 3. Survival of patients after HSCT after planned infusion of CAR T cells.
(A) Flow chart describing numbers of autologous and allogeneic patients enrolled in the 2 trials (NCT00968760 and NCT01497184, respectively). Enrollment occurred to obtain peripheral blood for manufacture of the genetically modified T cells and then the prospective patients were required to meet eligibility to administer the T cells. Some potential recipients were deemed ineligible to receive a successfully manufactured product, which highlights the medical fragility and advanced malignant disease of the enrollees. Some potential recipients were unable to receive T cells due to difficulties associated with their manufacture. All patients who received genetically modified T cells were included in survival analyses. Overall (blue line) and progression-free (red line) survival for (B) autologous as well as (C) allogeneic recipients and (D) the subset of allogeneic recipients from haploidentical donors.