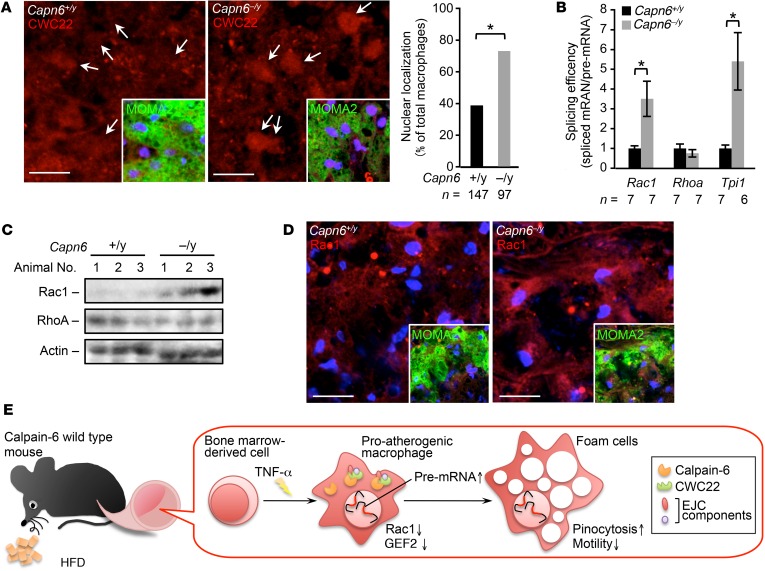
Figure 11. Loss of CAPN6 facilitates nuclear localization of CWC22 and subsequent Rac1 splicing in macrophages/foam cells in murine atheromas.
(A) Nuclear localization of CWC22 in macrophages in murine atheromas. MOMA2 served as a macrophage marker. Arrows represent nuclei. (B) Splicing of Cwc22 mRNA is accelerated by loss of Capn6. Splicing efficiency in whole aorta was measured by a PCR-based analysis. (C) Protein expression of Rac1 but not of RhoA in proatherogenic aorta. Protein expression in whole aorta was evaluated by immunoblotting. (D) Immunohistochemical distribution of Rac1 protein in macrophages in atheromas. MOMA2 served as a marker for macrophages. (E) Schematic depiction of the CAPN6-mediated disturbance of posttranscriptional regulation in proatherogenic macrophages. CAPN6 was induced in Capn6+/y macrophages in response to TNF-α and was associated with CWC22 in the cytoplasm. This association disturbs the nuclear localization of CWC22, thereby suppressing posttranscriptional processing of Rac1 and Arhgef2 (the genes encoding Rac1 and GEF2, respectively) and potentiates subsequent pinocytotic responses. Thus, CAPN6 induction in macrophages facilitates atherosclerotic development. *P < 0.05, Fisher’s exact test (A) and 1-way ANOVA followed by Bonferroni’s test (B); error bars represent mean ± SEM. Scale bars: 10 μm (A); 20 μm (D).