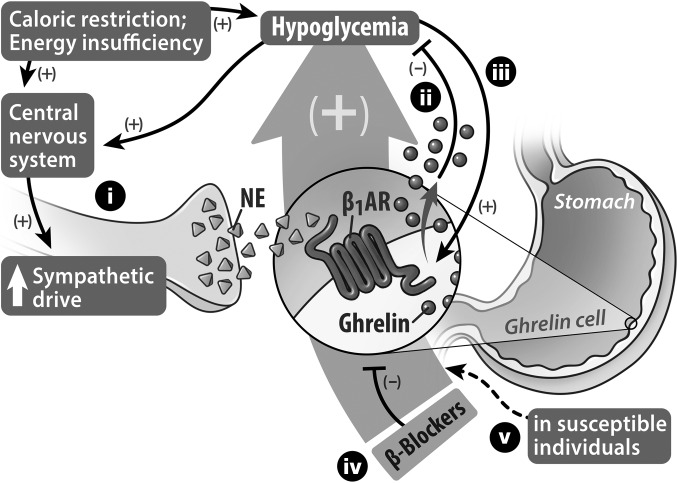
Figure 7. Proposed models of caloric restriction–induced ghrelin secretion to restore glucose homeostasis and of beta blocker–induced hypoglycemia.
(i) Caloric restriction acting through the CNS enhances sympathetic drive onto ghrelin cells that are distributed within the gastric mucosa. Norepinephrine released from the sympathetic nerve terminals activates β1ARs located on the ghrelin cells to induce ghrelin synthesis and secretion. (ii) In turn, the released acyl-ghrelin engages several processes, including GH secretion, that tend to limit falls in blood glucose. Reduced blood glucose can also stimulate a sympathetic response and, furthermore, (iii) can directly stimulate ghrelin cells to release ghrelin, although this direct stimulus only accounts for a fraction of the usual secretory response of ghrelin cells to caloric restriction. When body fat is depleted as a consequence of severe caloric restriction, the ghrelin cell β1AR–mediated release of ghrelin functions as a critical defense against marked falls in blood glucose, thereby promoting survival. (iv) Administration of beta blockers results in unintended blockade of ghrelin cell β1ARs, causing blunted ghrelin secretion. (v) When lack of normal ghrelin secretion caused by β1AR blockade is combined with food restriction, susceptible individuals, such as young children and probably also adults with cachexia or anorexia nervosa, become predisposed to developing life-threatening hypoglycemia.