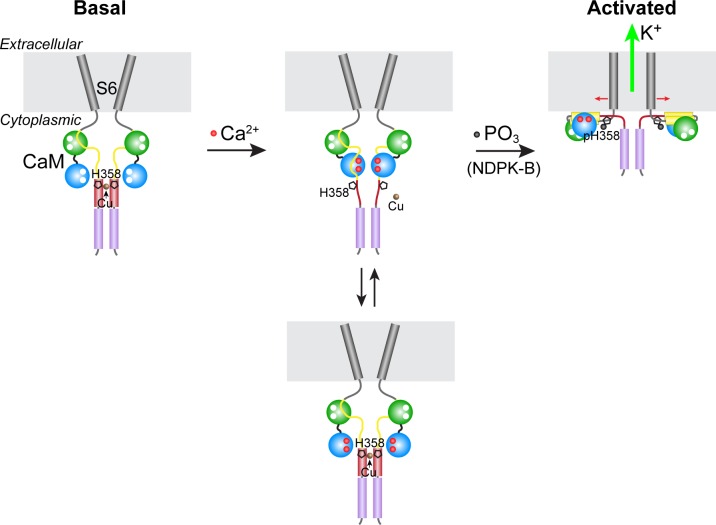
Figure 6. Model for KCa3.1 activation by calcium and histidine phosphorylation.
For clarity, only two of the four KCa3.1 subunits and calmodulin (CaM) are shown. In the basal state (no calcium; left panel), the CaM C lobe (green sphere) is bound to the N-terminal segment of the calmodulin-binding domain (CBD, yellow) in KCa3.1 (Schumacher et al., 2004). The transmembrane S6 helices close off the channel on the cytoplasmic side. At the C terminus of KCa3.1 is a coiled-coil region that forms a four-helix bundle (violet cylinders; S.R.H., unpublished data). Based on coiled-coil prediction software, it is probable that the region containing His358, which is just C-terminal to the CBD, also forms a four-helix bundle (maroon cylinders), with His358 (pentagon) occupying an inward-facing ‘a’ position in the heptad repeat. This would position the four copies of His358 for coordination of a Cu(II) ion on the axis of the four-helix bundle, which would stabilize the four-helix bundle and act to resist the conformational changes induced by calcium binding to the CaM N lobe (blue sphere). An increase in intracellular calcium induces conformational changes in the CBD that partially destabilize copper binding (middle panel), providing access to His358 for phosphorylation by NDPK-B (or copper chelation by TTM). Upon phosphorylation of His358, copper binding is abrogated, and the calcium-induced conformational changes in the CBD lead to channel opening (right panel; exact mechanism not known) (Adelman et al., 2012; Sachyani et al., 2014).