IgG4-related disease (IgG4-RD) is a multiorgan disorder that involves the salivary glands, pancreas and lungs [1]. We previously reported six patients with autoimmune pancreatitis (AIP) who showed characteristic central airway stenosis and bilateral hilar lymphadenopathy (BHL) that mimics sarcoidosis [2, 3]. We subsequently prospectively identified four additional patients with AIP and one patient with IgG4-related kidney disease (IgG4-RKD) with similar chest computed tomography (CT) findings and IgG4-positive plasma cell infiltration without granuloma in the airways. 10 out of 11 patients were diagnosed with AIP by their gastroenterologists based on the diagnostic AIP criteria proposed by the Japanese Pancreatic Society in 2011 [4] and the remaining one patient was diagnosed with IgG4-RKD by renal physicians based on the renal biopsy specimens in our hospital. All the 11 patients showed BHL and bronchial wall thickening on chest CT and were underwent transbronchial lung biopsy and bronchial biopsy according to our routine protocol [3]. The median IgG4-positive cell count and the percentage of IgG4-positive cells to IgG-positive plasma cells was 54.3 (range 17–80.6) per high-power field and 63.6% (range 34.5–96.5%), respectively. Thus, we diagnosed the 11 patients with IgG4-related respiratory disease (IgG4-RRD) characterised by airway involvement and BHL at multidisciplinary meetings of our hospital, as previously reported [3]. None of the 11 patients had oxygen desaturation and seven out of 11 patients had cough. The 10 patients with AIP showed one or more extrapulmonary manifestations other than in the pancreas and the one patient with IgG4-RKD had submandibular involvement. Nine patients with AIP received oral corticosteroid therapy for pancreatic lesions, whereas one patient did not because of the presence of severe cataract and glaucoma. The patient with IgG4-RKD did not receive oral corticosteroid therapy because spontaneous improvement of renal function was observed. Two patients received inhaled steroid therapy for cough. All 11 patients were alive at the end of our study and had no respiratory symptoms from these therapies.
Short abstract
BAL cytokines of IgG4-RRD patients are more characteristic of the Th2 response than those of sarcoidosis patients http://ow.ly/T2gDV
To the Editor:
IgG4-related disease (IgG4-RD) is a multiorgan disorder that involves the salivary glands, pancreas and lungs [1]. We previously reported six patients with autoimmune pancreatitis (AIP) who showed characteristic central airway stenosis and bilateral hilar lymphadenopathy (BHL) that mimics sarcoidosis [2, 3]. We subsequently prospectively identified four additional patients with AIP and one patient with IgG4-related kidney disease (IgG4-RKD) with similar chest computed tomography (CT) findings and IgG4-positive plasma cell infiltration without granuloma in the airways. 10 out of 11 patients were diagnosed with AIP by their gastroenterologists based on the diagnostic AIP criteria proposed by the Japanese Pancreatic Society in 2011 [4] and the remaining one patient was diagnosed with IgG4-RKD by renal physicians based on the renal biopsy specimens in our hospital. All the 11 patients showed BHL and bronchial wall thickening on chest CT and were underwent transbronchial lung biopsy and bronchial biopsy according to our routine protocol [3]. The median IgG4-positive cell count and the percentage of IgG4-positive cells to IgG-positive plasma cells was 54.3 (range 17–80.6) per high-power field and 63.6% (range 34.5–96.5%), respectively. Thus, we diagnosed the 11 patients with IgG4-related respiratory disease (IgG4-RRD) characterised by airway involvement and BHL at multidisciplinary meetings of our hospital, as previously reported [3]. None of the 11 patients had oxygen desaturation and seven out of 11 patients had cough. The 10 patients with AIP showed one or more extrapulmonary manifestations other than in the pancreas and the one patient with IgG4-RKD had submandibular involvement. Nine patients with AIP received oral corticosteroid therapy for pancreatic lesions, whereas one patient did not because of the presence of severe cataract and glaucoma. The patient with IgG4-RKD did not receive oral corticosteroid therapy because spontaneous improvement of renal function was observed. Two patients received inhaled steroid therapy for cough. All 11 patients were alive at the end of our study and had no respiratory symptoms from these therapies.
We assessed 33 consecutive patients diagnosed with sarcoidosis (stage I–II) [5] plus 11 consecutive patients with IgG4-RRD from September 2007 to March 2014 in our hospital. Sarcoidosis mainly involves the lungs and lymphatic system, and there are T-helper (Th)1 immune responses in affected organs [5]. In contrast, autoimmunity of IgG4-RD is widely regarded as the initial immunological stimulus for the Th2 immune response and Th2-cell responses are predominantly activated at affected sites [1]. There are few reports about the immune responses in the respiratory lesions of IgG4-RD. Here, we determined to compare the cytokine profiles in the bronchoalveolar lavage (BAL) fluid between IgG4-RRD and sarcoidosis for detecting the differences in these overlapping BHL diseases. All of the patients provided written informed consent for bronchoscopy and BAL fluid sampling. We measured the levels of 12 cytokines (interferon (IFN)-γ, interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13 and tumour necrosis factor (TNF)-α) in BAL fluid using the MILLIPLEX MAP Kit (Millipore, Darmstadt, Germany) and Luminex magnetic beads (Luminex, Austin, TX, USA) [6]. The Chi-squared test was used to compare the sex ratio and smoking status between IgG4-RRD and sarcoidosis, and the Mann–Whitney U-test was used to compare other results between the two groups (SPSS Statistics version 22; IBM, Armonk, NY, USA).
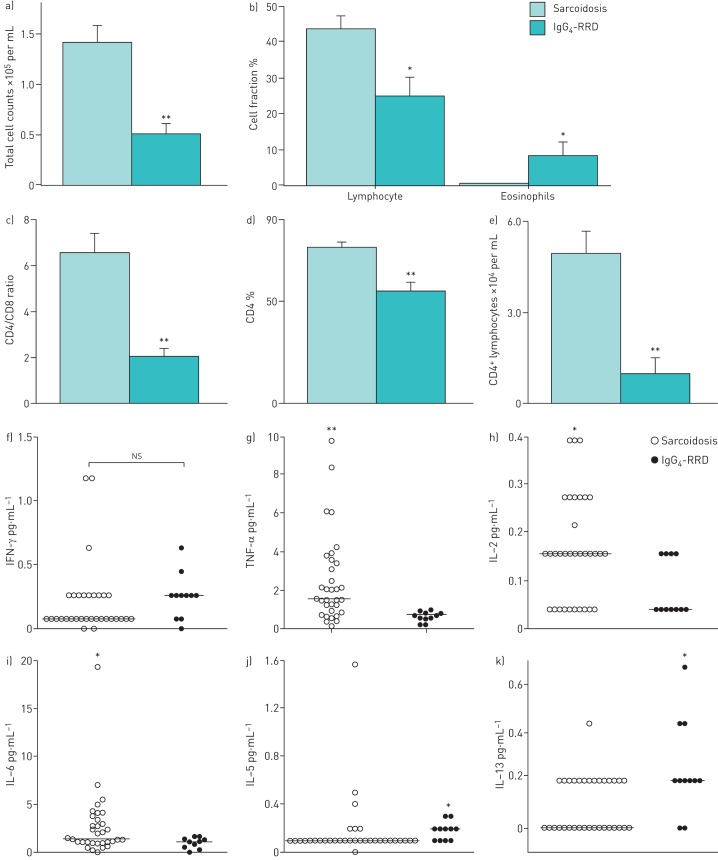
The 11 patients with IgG4-RRD (nine men and two women; median age 62 years, range 50–78 years) had a higher percentage of men (p<0.01) and were older (p<0.05) than the 33 patients with sarcoidosis (nine men and 24 women; median age 53 years, range 21–77 years). There was no statistical difference in smoker number or smoking status (IgG4-RRD: six never-smokers, two former smokers and three current smokers; sarcoidosis: 20 never-smokers, six former smokers and seven current smokers). All 44 patients showed BHL on chest CT. In the BAL fluid, the total cell counts and lymphocyte ratios were significantly higher in sarcoidosis (mean±se 14.09×105±1.63×105 cells per mL (p<0.01) and 44.12±3.58% (p<0.05), respectively) than in IgG4-RRD (4.97×105±1.15×105 cells per mL and 26.62±5.47%, respectively), and the eosinophil ratios were significantly higher in IgG4-RRD (8.86±4.46%) than in sarcoidosis (0.52±0.11%, p<0.05) (fig. 1a and b). The BAL fluid also showed significantly higher CD4 counts, CD4/CD8 ratios and CD4-positive lymphocyte counts in sarcoidosis (76.56±2.17%, 6.56±0.80 and 5.48×104±0.75×104 cells per mL) than in IgG4-RRD (54.71±4.21%, 2.03±0.32 and 1.23×104±0.27×104 cells per mL, respectively; p<0.01 for all comparisons) (fig. 1c–e). The concentrations of TNF-α, IL-2 and IL-6 in the BAL fluid were significantly higher in sarcoidosis (2.47±0.39, 0.16±0.01 and 2.69±0.59 pg·mL−1) than IgG4-RRD (0.66±0.07 (p<0.01), 0.08±0.01 (p<0.05) and 0.89±0.17 pg·mL−1 (p<0.05), respectively) (fig. 1g–i). In contrast, the concentrations of IL-5 and IL-13 were significantly higher in IgG4-RRD (0.18±0.02 and 0.24±0.06 pg·mL−1) than in sarcoidosis (0.17±0.04 and 0.09±0.02 pg·mL−1, both p<0.05) (fig. 1j and k), and IL-4 showed the same tendency. There were no significant differences in the concentrations of IFN-γ (fig. 1f) or other cytokines between the two groups.
FIGURE 1.
a) Total cell counts, b) lymphocyte and eosinophil percentages, c) CD4/CD8 ratios, d) proportion of CD4-positive cells and e) CD4-positive lymphocyte counts in the bronchoalveolar lavage (BAL) fluid of patients with sarcoidosis (n=33) and patients with IgG4-related disease (IgG4-RD) (n=11). Data are presented as mean±se. f) Interferon (IFN)-γ, g) tumour necrosis factor (TNF)-α, h) interleukin (IL)-2, i) IL-6, j) IL-5 and k) IL-13 concentrations in the BAL fluids of patients with sarcoidosis (n=33) and patients with IgG4-RD (n=11). Bars indicate the median value. ns: not significant. *: p<0.05 (Mann–Whitney U-test); **: p<0.01 (Mann–Whitney U-test).
The term IgG4-RRD is broad and does not capture the specific airway-centred disease; however, BHL and thickening of bronchial wall/ bronchovascular bundle are common CT findings in IgG4-RRD [7]. It was difficult to distinguish the 11 patients with IgG4-RRD from the 33 patients with sarcoidosis using radiological findings alone. Most of the patients with IgG4-RRD in this study were middle-aged to elderly men, as reported previously [7]. In the BAL fluid, sarcoidosis showed stronger CD4 lymphocyte responses than IgG4-RRD. Generally in sarcoidosis, the granuloma is characterised by a core of monocyte-derived epithelioid histiocytes and multinucleated giant cells with interspersed CD4-positive T-lymphocytes [8]. Therefore, both IgG4-RRD and sarcoidosis are on the CD4 lymphocyte dominant disease spectrum. Although the measurement of BAL fluid cytokines cannot be used routinely in the clinical practice, we attempted to measure the cytokines to verify the difference in the phenotype of the CD4 lymphocytes. Namely, the BAL fluid concentrations of Th1 cytokines (IL-2, IL-6 and TNF-α) were significantly higher in sarcoidosis. Conversely, the BAL fluid concentrations of Th2 cytokines, such as IL-5 and IL-13, were significantly higher in IgG4-RRD. Walker et al. [9] analysed the cytokine profile in the BAL fluid of sarcoidosis and found a Th1-cell cytokine pattern characterised by increased concentrations of IL-2 and IFN-γ and normal levels of IL-4 and IL-5. Prasse et al. [10] also reported that Th1-like cytokines, such as IL-2, IFN-γ and TNF-α, are markedly increased in the BAL fluids of patients with sarcoidosis compared with normal controls but that, in contrast, IL-4 and IL-13 show similar levels in the two groups. Here, we found a predominant Th2 immune response in IgG4-RRD plus eosinophil infiltrates that depend on Th2 cytokines.
The pathogenesis and immunological features of IgG4-RD are not fully understood. A pathology study using real-time PCR found higher levels of Th2 cytokines (IL-4, IL-5 and IL-13) in affected lesions of the pancreas, bile ducts, lachrymal glands and salivary glands of IgG4-RD patients than in patients with autoimmune biliary diseases such as primary sclerosing cholangitis and primary biliary cirrhosis [11]. In ductal lesions, the bile samples from patients with IgG4-related cholangitis showed significant increases in the levels of the Th2 cytokines IL-4 and IL-5 [12]. The cytokine profiles in our BAL fluid samples from IgG4-RRD patients reflected that the inflammatory conditions of the lungs are consistent with cytokine profiles of extrapulmonary lesions in IgG4-RD, described in previous reports [1, 11, 12]. Interestingly, the concentrations of IFN-γ in IgG4-RRD BAL fluid were not lower than in sarcoidosis in this study. Zen et al. [11] reported that in the extrapulmonary lesions, the expression of IFN-γ in the tissues affected by IgG4-RD, including pancreas, bile duct and salivary glands, was also comparable to that in other Th1-dominant autoimmune diseases such as primary sclerosing cholangitis and primary biliary cirrhosis. Okazaki et al. [13] also reported that in the peripheral blood of IgG4-RD patients, the number of IFN-γ-producing peripheral CD4-positive cells and the secreted level of IFN-γ are significantly higher than in controls. These data and ours suggest that the Th1/Th2 balance seems shifted towards Th2 in IgG4-RD; however, Th1-type cytokines such as IFN-γ are not suppressed at affected sites.
To our knowledge, this is the largest series of patients with airway involvement in IgG4-RD with BHL that is similar to sarcoidosis, and the first study of cytokines in the BAL fluid of IgG4-RD patients. To summarise, the cytokine profiles in the BAL fluid of IgG4-RRD were more characteristic of the Th2 response compared with sarcoidosis.
Acknowledgement
We thank Shigeyuki Kawa (Center for Health, Safety and Environmental Management, Shinshu University School of Medicine, Matsumoto, Japan) for expert advice and management of some of the IgG4-RD patients. We thank Hitomi Imamura (First Department of Internal Medicine, Shinshu University School of Medicine, Matsumoto, Japan) and Akane Sueki (Department of Laboratory Medicine, Shinshu University School of Medicine, Matsumoto, Japan) for skilled technical assistance with the cytokine measurements, and Yunden Droma (First Department of Internal Medicine, Shinshu University School of Medicine, Matsumoto, Japan) for help in manuscript preparation.
Footnotes
Support statement: This study was funded by an Intractable Disease grant from the Health and Labor Sciences Research Grants of the Ministry of Health, Labor and Welfare (Tokyo, Japan). Funding information for this article has been deposited with FundRef.
Conflict of Interest: None declared.
References
- 1.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med 2012; 366: 539–551. [DOI] [PubMed] [Google Scholar]
- 2.Ito M, Yasuo M, Yamamoto H, et al. . Central airway stenosis in a patient with autoimmune pancreatitis. Eur Respir J 2009; 33: 680–683. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto H, Yasuo M, Ito M, et al. . Clinical features of central airway involvement in autoimmune pancreatitis. Eur Respir J 2011; 38: 1233–1236. [DOI] [PubMed] [Google Scholar]
- 4.Okazaki K, Kawa S, Kamisawa T, et al. . Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 I. Concept and diagnosis of autoimmune pancreatitis. J Gastroenterol 2014; 49: 567–588. [DOI] [PubMed] [Google Scholar]
- 5.Valeyre D, Prasse A, Nunes H, et al. . Sarcoidosis. Lancet 2014; 383: 1155–1167. [DOI] [PubMed] [Google Scholar]
- 6.Moncunill G, Aponte JJ, Nhabomba AJ, et al. . Performance of multiplex commercial kits to quantify cytokine and chemokine responses in culture supernatants from Plasmodium falciparum stimulations. PLoS One 2013; 8: e52587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsui S, Hebisawa A, Sakai F, et al. . Immunoglobulin G4-related lung disease: clinicoradiological and pathological features. Respirology 2013; 18: 480–487. [DOI] [PubMed] [Google Scholar]
- 8.Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med 2011; 183: 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker C, Bauer W, Braun RK, et al. . Activated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophilia. Am J Respir Crit Care Med 1994; 150: 1038–1048. [DOI] [PubMed] [Google Scholar]
- 10.Prasse A, Georges CG, Biller H, et al. . Th1 cytokine pattern in sarcoidosis is expressed by bronchoalveolar CD4+ and CD8+ T cells. Clin Exp Immunol 2000; 122: 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zen Y, Fujii T, Harada K, et al. . Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology 2007; 45: 1538–1546. [DOI] [PubMed] [Google Scholar]
- 12.Müller T, Beutler C, Picó AH, et al. . Increased T-helper 2 cytokines in bile from patients with IgG4-related cholangitis disrupt the tight junction-associated biliary epithelial cell barrier. Gastroenterology 2013; 144: 1116–1128. [DOI] [PubMed] [Google Scholar]
- 13.Okazaki K, Uchida K, Ohana M, et al. . Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology 2000; 118: 573–581. [DOI] [PubMed] [Google Scholar]