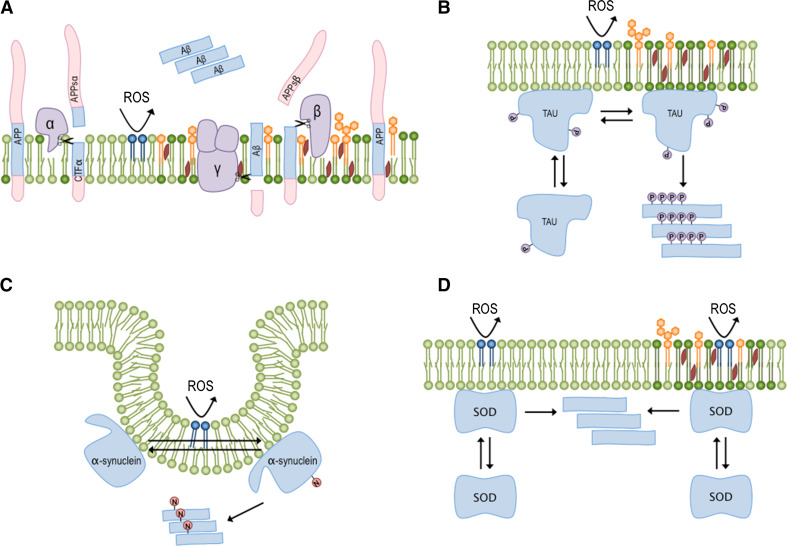
Fig. 1.
Inflammation can disturb the membrane and promote protein aggregation. Activated microglia cells produce peptides, proteins and reactive species of oxygen and nitrogen that can perturb membrane homeostasis. The binding of peptides and the oxidation of phospholipids can increase the gel phase and raft domains, triggering protein aggregation and disease. a APP is in equilibrium between its native and cleaved forms, the latter of which is generated by the action of different secretases (purple). In the non-lipid raft portion, which is abundant in unsaturated phospholipids (light green), cleavage by α-secretase produces soluble APP and membrane-bound CTFα. However, in terms of lipid rafts, where the membrane domains are rich in cholesterol (red), glycosphingolipids (orange) and saturated lipids (dark green), APP cleavage by β-secretase can generate APP-sβ and CTFβ. The latter peptide can subsequently be processed by γ-secretase to produce the aggregation prone Aβ peptide (blue). b Changes in membrane composition can modify the phosphorylation pattern (purple circles) of the tau protein and promote its aggregation propensity. Aβ aggregates and tau neurofibrillar tangles are characteristic of AD. c Alterations in the distribution of α-synuclein between different membrane domains can trigger a self-assembling process, which can be accelerated by lipid peroxidation (dark blue). α-synuclein oligomers can damage neuron membranes and promote the development of PD. d Superoxide dismutase (SOD) can also bind to different membrane domains in different compositions, and its distribution between them can modulate its aggregation propensity. Specifically, an increase in the content of saturated fatty acids and oxidized lipids can support SOD-oligomerization, thereby promoting the development of ALS