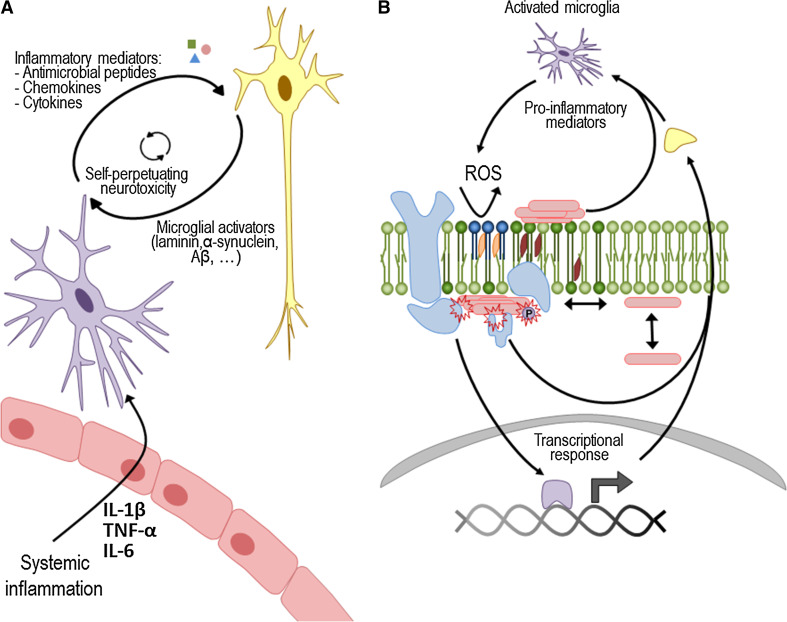
Fig. 2.
Membrane damage as an explanation on how microglia-secreted mediators may promote protein aggregation. a When a systemic infection is detected in the body, several inflammatory mediators are released. Some of these can cross the blood–brain barrier and stimulate microglial cells (purple). These cells change its morphology and become activated, releasing pro-inflammatory cytokines, ROS and other peptide mediators in the brain. When a chronic inflammatory signal is present, microglia cells become permanently primed, and the continued release of pro-inflammatory mediators can damage the surrounding neuronal cells (yellow). In turn, neuronal damage contributes to increasing the microglia activators that further activate microglial cells, causing a dangerous, self-sustaining activation cycle. b At the molecular level, ROS and the binding of pro-inflammatory mediators to specific receptors generate a transcriptional response in neurons. Moreover, ROS can cause lipid peroxidation (dark blue), thus perturbing neuronal membrane homeostasis, while the unspecific binding of peptide mediators can alter membrane fluidity. Globally, the integrated response to microglial secretions at the membrane level may lead to aggregation and protein mislocation. This may also trigger changes in post-translational modifications, including the phosphorylation state of several proteins such as α-synuclein, Aβ and tau. Last but not least, protein aggregates themselves or through the activation of receptors and signaling complexes (e.g. TLR2 or NLRP3 inflammasome) may generate a self-perpetuating mechanism of increased membrane damage and aggregation