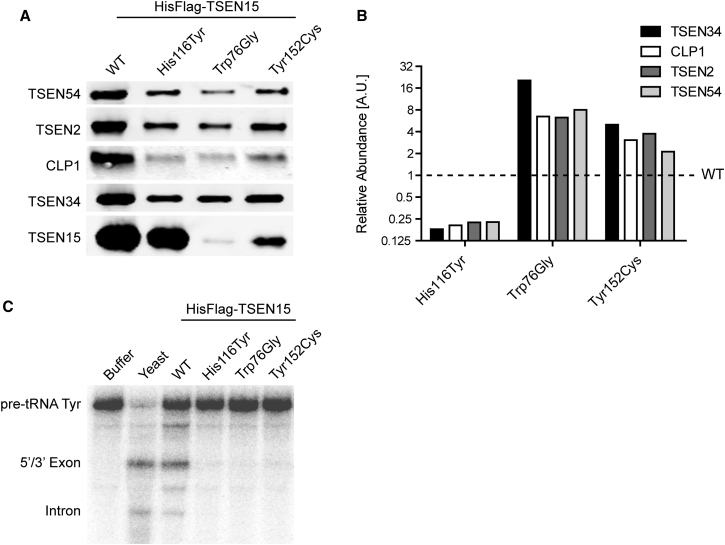
Figure 3.
The Three Described Amino Acid Substitutions Affect Complex Assembly and tRNA Cleavage
(A) Western blot analysis of purified samples obtained from stable cell lines expressing wild-type (WT) or mutant HisFlag-tagged TSEN15. All lysates were subjected to tandem affinity purification (Ni-NTA and Flag M2, Sigma, cat# F1804, RRID: AB_262044) and probed with antibodies detecting TSEN54 (Abcam, ab178696, 0.86 μg/mL), TSEN2 (Proteintech, cat# 13103-2-AP, RRID: AB_2272213; 4 μg/mL), CLP1 (HEAB antibody, Abcam, ab172683, 0.4 μg/mL), TSEN34 (Abcam, ab68868, 1 μg/mL), and TSEN15 (Flag M2, Sigma, cat# F1804, RRID: AB_262044; 0.2 μg/mL). Whereas the p.His116Tyr mutant TSEN15 can be recovered at levels comparable to WT, p.Trp76Gly and p.Tyr152Cys result in lower protein levels and affect protein folding, stability, or both.
(B) Quantification of TSEN54, TSEN2, CLP1, and TSEN34 levels, normalized to the respective mutant TSEN15 and wild-type (equals 1). 1:1:1:1 stoichiometry of the complex, as well as relative levels of CLP1, are severely affected by the substitutions. Whereas the p.His116Tyr substitution results in destabilization of the purified complex and relative reduction of its components and CLP1 protein, the other two mutants show a relative increase of the other proteins.
(C) tRNA cleavage assay using 32P-labeled tyrosine pre-tRNA (pre-tRNA Tyr) as substrate. Shown is an RNA gel of reactions incubated with buffer, purified yeast complex (Yeast), the purified WT complex, and the mutants. Yeast, as well as WT complex, catalyze a cleavage reaction that results in the production of a 5′ and a 3′ exon, as well as the intron. All three mutant complexes show near complete loss of cleavage activity.