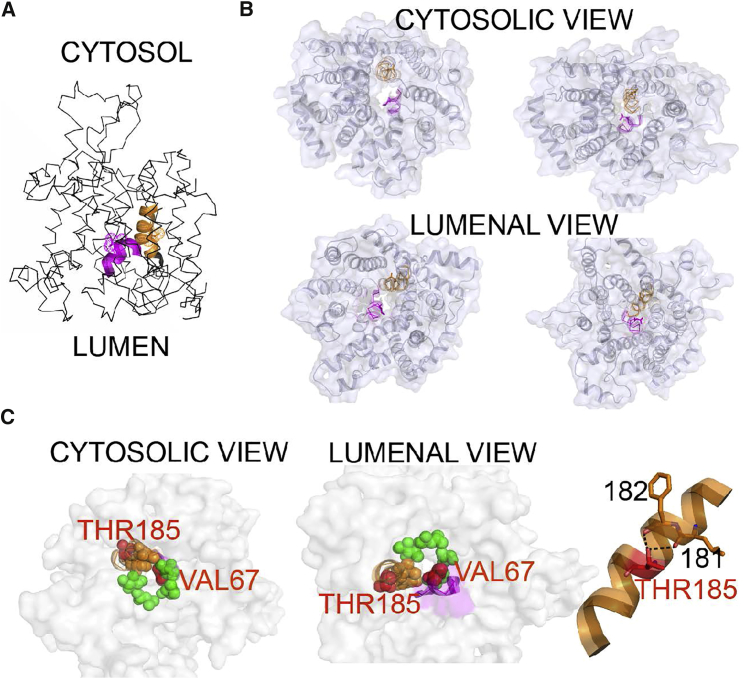
Figure 3.
Structural Topology of the Mutations in Sec61
The cryo-EM based models of translocons translating hydrophilic peptide with opened pore (PDB: 4CG5) and translating peptide inserted to membrane possessing a pore sealed by a plug domain (PDB: 4CG6) were used for illustration. Regions containing mutations are depicted with cartoon representation, namely plug domain (p.Val67Gly) in magenta and TM5 (p.Thr185Val) in orange. Mutated residues are highlighted as dots and/or sticks.
(A) Overall structure of Sec61 and location of the mutated residues.
(B) Orientation of the mutated residues in the Sec61 structure with opened translocation pore (left) and with pore sealed by a plug domain (right).
(C) Left: constriction ring formed by apolar residues represented by green (TM2, TM7, and TM10) and orange (positioned at TM5 near Thr185 residue) spheres; mutated residues are shown as red spheres. Right: detail of TM5 illustrating hydrogen bonding of hydroxyl Thr185 with carbonyls of Leu181 and Phe182.