Abstract
AIMS: To obtain reference values of the level of expression of T cell antigens on normal lymphocyte subsets in order to disclose differences which could reflect their function or maturation stages, or both. METHODS: Peripheral blood from 15 healthy donors was processed by flow cytometry with triple colour analysis. For each sample phycoerythrin (PE) conjugated CD2, CD4, CD5, CD8, and CD56 monoclonal antibodies were combined with Cy5-R-phycoerythrin (TC) conjugated CD3 and fluorescein isothiocyanate (FITC) conjugated CD7; CD2- and CD7-PE were also combined with CD3-TC and CD4-FITC. Standard microbeads with different capacities to bind mouse immunoglobulins were used to convert the mean fluorescence intensity (MFI) values of the lymphocyte subsets identified by multiparametric flow cytometry into the number of antigen molecules per cell, measured as antibody binding capacity (ABC). RESULTS: CD4+ (helper/inducer) T cells exhibit a higher CD3 antigen expression compared with CD8+ (suppressor/ cytotoxic) T lymphocytes. Within the CD4+ T cells, the CD4+CD7- subset expressed a lower level of CD3 compared with CD4+CD7+ and CD8+CD7+ cells, and higher CD2 and CD5 expression than the main CD3+CD7+ subset. Major differences in antigen expression were also detected between CD3+ T cells and CD3-CD56+ natural killer (NK) cells: NK cells exhibited higher levels of CD7 and CD56 and lower levels of CD2 and CD5 than T cells. Significantly lower CD5 expression was also detected in the small CD5+ B lymphocyte subset compared with T cells. CONCLUSIONS: Quantitative flow cytometry with triple colour analysis may be used to detect antigen modulations in disease states and to increase the accuracy of diagnosis by comparison with findings in normal counterparts.
Full text
PDF

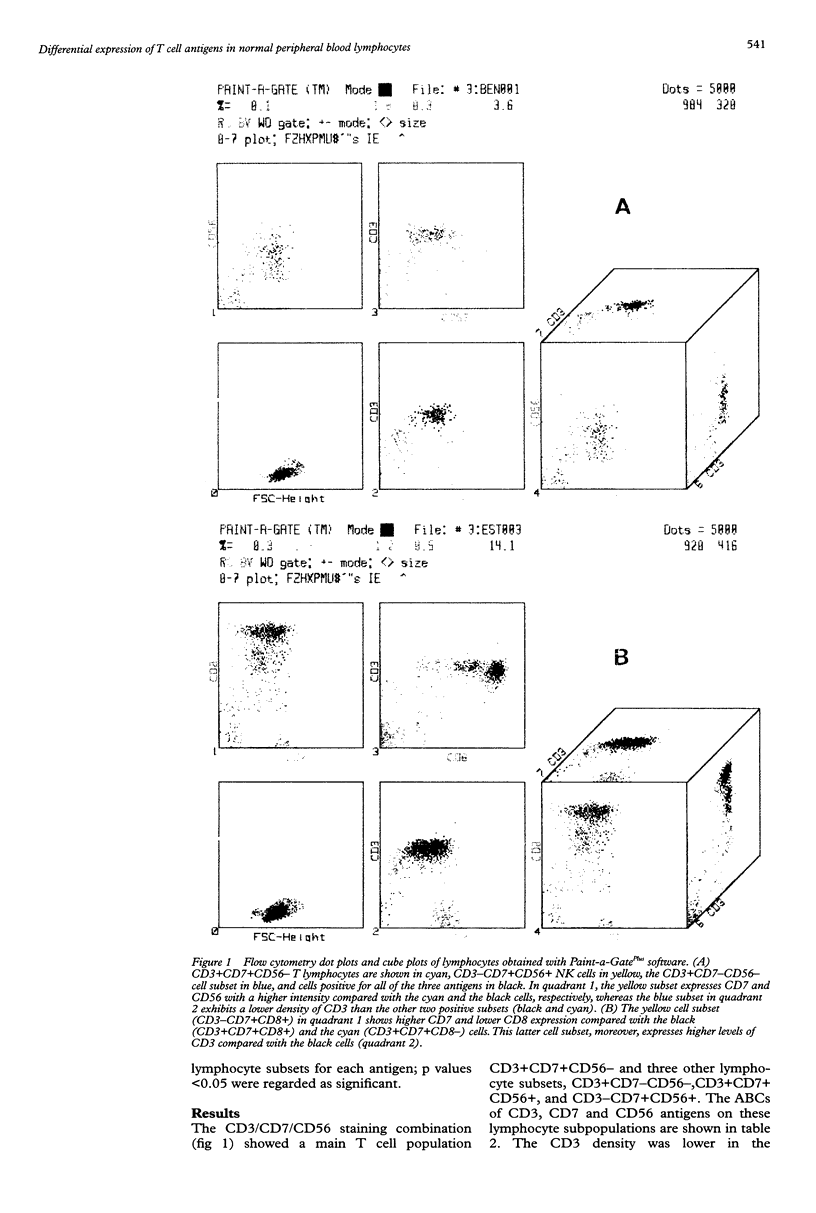
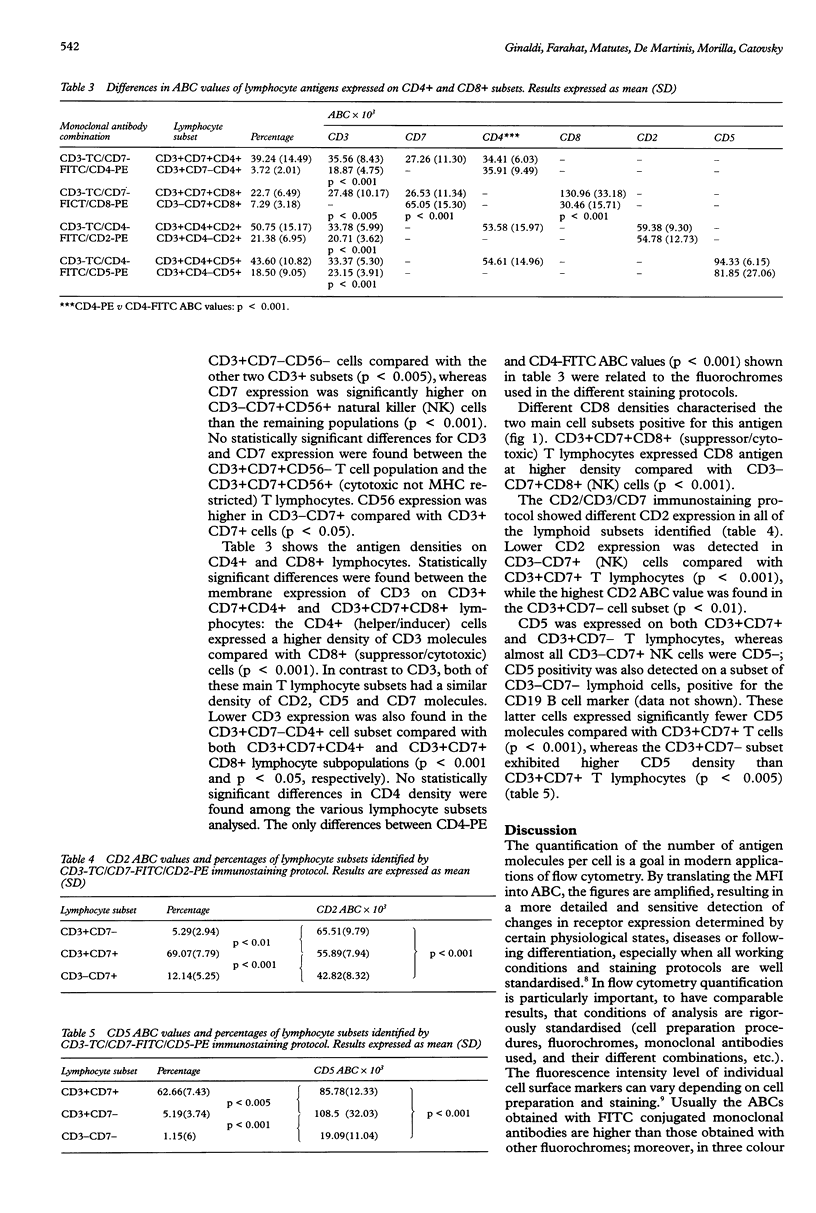


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Caligiuri M., Ritz J., Schlossman S. F. CD3-negative natural killer cells express zeta TCR as part of a novel molecular complex. Nature. 1989 Sep 14;341(6238):159–162. doi: 10.1038/341159a0. [DOI] [PubMed] [Google Scholar]
- Beverley P. The importance of T3 in the activation of T lymphocytes. Nature. 1983 Aug 4;304(5925):398–399. doi: 10.1038/304398b0. [DOI] [PubMed] [Google Scholar]
- Bierer B. E., Burakoff S. J. T-lymphocyte activation: the biology and function of CD2 and CD4. Immunol Rev. 1989 Oct;111:267–294. doi: 10.1111/j.1600-065x.1989.tb00549.x. [DOI] [PubMed] [Google Scholar]
- Bárcena A., Muench M. O., Galy A. H., Cupp J., Roncarolo M. G., Phillips J. H., Spits H. Phenotypic and functional analysis of T-cell precursors in the human fetal liver and thymus: CD7 expression in the early stages of T- and myeloid-cell development. Blood. 1993 Dec 1;82(11):3401–3414. [PubMed] [Google Scholar]
- Chiron M., Darbon J. M., Roubinet F., Cassar G., Jaffrezou J. P., Bordier C., Laurent G. Quantitative analysis of CD5 antigen modulation by 12-O-tetradecanoylphorbol-13-acetate in T-lymphoblastic leukemia cells: individual response patterns and their relationships with both maturation and protein kinase C content. Cell Immunol. 1990 Oct 15;130(2):339–351. doi: 10.1016/0008-8749(90)90277-x. [DOI] [PubMed] [Google Scholar]
- Farahat N., Lens D., Morilla R., Matutes E., Catovsky D. Differential TdT expression in acute leukemia by flow cytometry: a quantitative study. Leukemia. 1995 Apr;9(4):583–587. [PubMed] [Google Scholar]
- Farahat N., Lens D., Zomas A., Morilla R., Matutes E., Catovsky D. Quantitative flow cytometry can distinguish between normal and leukaemic B-cell precursors. Br J Haematol. 1995 Nov;91(3):640–646. doi: 10.1111/j.1365-2141.1995.tb05360.x. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Martin M. E., Kay H. H., Kurtzberg J. Early events in human T cell ontogeny. Phenotypic characterization and immunohistologic localization of T cell precursors in early human fetal tissues. J Exp Med. 1988 Sep 1;168(3):1061–1080. doi: 10.1084/jem.168.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T., Phillips J. H., Duncan B., Lanier L. L., Spits H. Human fetal liver-derived CD7+CD2lowCD3-CD56- clones that express CD3 gamma, delta, and epsilon and proliferate in response to interleukin-2 (IL-2), IL-3, IL-4, or IL-7: implications for the relationship between T and natural killer cells. Blood. 1992 Sep 1;80(5):1270–1278. [PubMed] [Google Scholar]
- Islam D., Lindberg A. A., Christensson B. Peripheral blood cell preparation influences the level of expression of leukocyte cell surface markers as assessed with quantitative multicolor flow cytometry. Cytometry. 1995 Jun 15;22(2):128–134. doi: 10.1002/cyto.990220208. [DOI] [PubMed] [Google Scholar]
- Kukel S., Reinhold U., Oltermann I., Kreysel H. W. Progressive increase of CD7- T cells in human blood lymphocytes with ageing. Clin Exp Immunol. 1994 Oct;98(1):163–168. doi: 10.1111/j.1365-2249.1994.tb06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R., Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- Lavabre-Bertrand T., Duperray C., Brunet C., Poncelet P., Exbrayat C., Bourquard P., Lavabre-Bertrand C., Brochier J., Navarro M., Janossy G. Quantification of CD24 and CD45 antigens in parallel allows a precise determination of B-cell maturation stages: relevance for the study of B-cell neoplasias. Leukemia. 1994 Mar;8(3):402–408. [PubMed] [Google Scholar]
- Lavabre-Bertrand T., Janossy G., Exbrayat C., Bourquard P., Duperray C., Navarro M. Leukemia-associated changes identified by quantitative flow cytometry. II. CD5 over-expression and monitoring in B-CLL. Leukemia. 1994 Sep;8(9):1557–1563. [PubMed] [Google Scholar]
- Lavabre-Bertrand T., Janossy G., Ivory K., Peters R., Secker-Walker L., Porwit-MacDonald A. Leukemia-associated changes identified by quantitative flow cytometry: I. CD10 expression. Cytometry. 1994 Dec 15;18(4):209–217. doi: 10.1002/cyto.990180404. [DOI] [PubMed] [Google Scholar]
- Legac E., Autran B., Merle-Beral H., Katlama C., Debre P. CD4+CD7-CD57+ T cells: a new T-lymphocyte subset expanded during human immunodeficiency virus infection. Blood. 1992 Apr 1;79(7):1746–1753. [PubMed] [Google Scholar]
- Lenkei R., Andersson B. Determination of the antibody binding capacity of lymphocyte membrane antigens by flow cytometry in 58 blood donors. J Immunol Methods. 1995 Jun 28;183(2):267–277. doi: 10.1016/0022-1759(95)00064-h. [DOI] [PubMed] [Google Scholar]
- Luo W., Van de Velde H., von Hoegen I., Parnes J. R., Thielemans K. Ly-1 (CD5), a membrane glycoprotein of mouse T lymphocytes and a subset of B cells, is a natural ligand of the B cell surface protein Lyb-2 (CD72). J Immunol. 1992 Mar 15;148(6):1630–1634. [PubMed] [Google Scholar]
- McCarthy D. A., Macey M. G., Cahill M. R., Newland A. C. Effect of fixation on quantification of the expression of leucocyte function-associated surface antigens. Cytometry. 1994 Sep 1;17(1):39–49. doi: 10.1002/cyto.990170106. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Acuto O., Hussey R. E., Hodgdon J. C., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Evidence for the T3-associated 90K heterodimer as the T-cell antigen receptor. Nature. 1983 Jun 30;303(5920):808–810. doi: 10.1038/303808a0. [DOI] [PubMed] [Google Scholar]
- Miller J. S., Verfaillie C., McGlave P. The generation of human natural killer cells from CD34+/DR- primitive progenitors in long-term bone marrow culture. Blood. 1992 Nov 1;80(9):2182–2187. [PubMed] [Google Scholar]
- Moretta A., Bottino C., Pende D., Tripodi G., Tambussi G., Viale O., Orengo A., Barbaresi M., Merli A., Ciccone E. Identification of four subsets of human CD3-CD16+ natural killer (NK) cells by the expression of clonally distributed functional surface molecules: correlation between subset assignment of NK clones and ability to mediate specific alloantigen recognition. J Exp Med. 1990 Dec 1;172(6):1589–1598. doi: 10.1084/jem.172.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnes J. R. Molecular biology and function of CD4 and CD8. Adv Immunol. 1989;44:265–311. doi: 10.1016/s0065-2776(08)60644-6. [DOI] [PubMed] [Google Scholar]
- Prince H. E., Bermudez S., Plaeger-Marshall S. Preparation of CD8bright and CD8dim lymphocyte populations using two positive selection methods in tandem. J Immunol Methods. 1993 Oct 15;165(2):139–148. doi: 10.1016/0022-1759(93)90339-9. [DOI] [PubMed] [Google Scholar]
- Rabinowich H., Pricop L., Herberman R. B., Whiteside T. L. Expression and function of CD7 molecule on human natural killer cells. J Immunol. 1994 Jan 15;152(2):517–526. [PubMed] [Google Scholar]
- Reinhold U., Abken H., Kukel S., Moll M., Müller R., Oltermann I., Kreysel H. W. CD7- T cells represent a subset of normal human blood lymphocytes. J Immunol. 1993 Mar 1;150(5):2081–2089. [PubMed] [Google Scholar]
- Schmidt-Wolf I. G., Lefterova P., Johnston V., Huhn D., Blume K. G., Negrin R. S. Propagation of large numbers of T cells with natural killer cell markers. Br J Haematol. 1994 Jul;87(3):453–458. doi: 10.1111/j.1365-2141.1994.tb08297.x. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Fernández-Repollet E. Development of clinical standards for flow cytometry. Ann N Y Acad Sci. 1993 Mar 20;677:28–39. doi: 10.1111/j.1749-6632.1993.tb38760.x. [DOI] [PubMed] [Google Scholar]
- Sutherland D. R., Rudd C. E., Greaves M. F. Isolation and characterization of a human T lymphocyte-associated glycoprotein (gp40). J Immunol. 1984 Jul;133(1):327–333. [PubMed] [Google Scholar]



