Abstract
Recommendations for prevention of cardiovascular diseases (CVDs) risk factors among older adults highlighted the importance of exercise-based interventions, including endurance training (ET). However, the evidence of efficacy of other interventions based on short-bouts of exercise (circuit training, CT), and the practice of breath-control and meditation (relaxing training, RT) is growing. The aim of this study was to elucidate if CT or RT are equally effective in CVD risk factors reduction compared to ET. To this purpose, in 40 elderly participants, with clinically diagnosed grade 1 hypertension, resting blood pressure, blood glucose, and cholesterol levels, peak oxygen uptake (), mechanical efficiency and quality of life were evaluated before and after 12 weeks of ET, CT, and RT treatments. Resting blood pressure reduced significantly in all groups by ∼11 %. In ET, blood cholesterol levels (−18 %), (+8 %), mechanical efficiency (+9 %), and quality of life scores (+36 %) ameliorated. In CT blood glucose levels (−11 %), (+7 %) and quality of life scores (+35 %) were bettered. Conversely, in RT, the lower blood pressure went along only with an improvement in the mental component of quality of life (+42 %). ET and CT were both appropriate interventions to reduce CVDs risk factors, because blood pressure reduction was accompanied by decreases in blood glucose and cholesterol levels, increases in , mechanical efficiency, and quality of life. Although RT influenced only blood pressure and quality of life, this approach would be an attractive alternative for old individuals unable or reluctant to carry out ET or CT.
Keywords: Aging, Blood pressure, Cardiovascular disease, Circuit training, Endurance training, Relaxing training
Introduction
Cardiovascular diseases (CVDs) are the main cause of mortality and the most common reason of permanent disability in the western countries (Go et al. 2013). Several risk factors contribute to the development of CVDs, such as age, elevated levels of blood cholesterol, diabetes, obesity, tobacco use, sedentary life style, and hypertension (HYP; Najjar et al. 2005). When HYP and other CVD risk factors are concomitantly present, they may potentiate each other leading to a greater total cardiovascular risk than the sum of its individual components (Lewington et al. 2002; Mancia et al. 2013a). In the view of this close correlation among CVD risk factors, the updated recommendations for prevention of CVDs highlight the importance of physical activity among treatments of HYP (Aronow et al. 2011; Mancia et al. 2013b). Specifically, hypertensives are encouraged to participate in endurance training (ET) programs to reduce both systolic and diastolic arterial blood pressure. This ET-induced reduction in arterial pressure is generally accompanied by improvements in central and peripheral hemodynamic factors (Gibala et al. 2012; Hood et al. 2011). Additional benefits of ET are the improvement of health-related quality of life (Cadore et al. 2015), the enhancement in maximal aerobic capacity (), which is highly correlated with longevity and independence of aged population (Venturelli et al. 2012a, b), and the reduction of other CVDs risk factors, such as glucose and cholesterol levels, and obesity (Pescatello et al. 2004)
Also other emerging exercise-based treatments for HYP may reduce resting arterial blood pressure (Pal et al. 2013; Sousa et al. 2013), and improve health-related quality of life (Romero-Arenas et al. 2013). Interestingly, circuit-training (CT) physical exercise is becoming one of the most popular fitness programs in healthy old individuals because of its greater enjoyment with respect to standard ET (Bartlett et al. 2011). However, limited data are available on the effectiveness of this exercise approach on the reduction of CVDs risk factors in old hypertensives (Guimaraes et al. 2010; Lamina 2010). Besides the potential appeal of CT for the old population, it is important to note that the physiological mechanisms activated by CT are different to those involved in ET in terms of central hemodynamics stimulation (Zhang et al. 2014). The limited heart rate (HR) and cardiac output responses are indeed counterbalanced by high stimulation of the peripheral circulation as during small muscle mass exercise (Esposito et al. 2010, 2011).
Remarkably, a reduction in arterial blood pressure and the amelioration of health-related quality of life (Oken et al. 2006) has been observed also after training based on breath control and meditation (relaxing training, RT) (Patel 1975; Santaella et al. 2006). RT is a simple method to improve autonomic balance, respiratory control, and, consequently, to reduce blood pressure in hypertensive individuals. However, whether this slow-breath training can also ameliorate other CVDs risk factors is still a matter of investigation.
Given the clear evidence of ET effectiveness on the amelioration of several CVDs risk factors, exercise capacity, and quality of life in elderly patients with HYP, the aim of this study was to elucidate if other exercise-based interventions, based upon CT (mainly peripheral stimulation) or RT (no central or peripheral hemodynamic involvement), are equally successful with respect to ET (both central and peripheral stimulation). To this purpose, resting blood pressure, blood glucose and cholesterol levels, maximal exercise capacity, mechanical efficiency, and quality of life were evaluated before and after 12 weeks of ET, CT, and RT treatments. We hypothesized that a similar positive effect on blood pressure and health-related quality of life will be retrieved in all conditions. However, this expected outcome will be complemented with positive effects on blood glucose and cholesterol levels, maximal exercise capacity, and mechanical efficiency only in ET and CT, but not in RT.
Methods
Participants
Forty participants (20 males and 20 females; age range 65–74 years), with clinically diagnosed grade 1 hypertension corresponding to a mean systolic and diastolic blood pressure of 140–159 and/or 90–99 mmHg, respectively, volunteered in the study and signed a written informed consent form. This range of age has been chosen because the prevalence of HYP is elevated by ∼55 %, and equally balanced between males and females (Reckelhoff 2001). The old hypertensive volunteers were recruited among home resident older adults. Three general-medicine physicians from the same district recruited potential participants from their database. At the time of recruitment, all the participants were chronically sedentary (Table 1) and most of them never practiced sports during their life. All procedures conformed to the standards set by the 1974 Declaration of Helsinki, and the Institutional Review Boards of the local University approved the study. Patients with beta-blockers were excluded from the study. Participants’ medications were not altered throughout the investigation. Participants’ characteristics, the medications taken, and comorbidities are listed in Table 1.
Table 1.
Participants’ characteristics
| Group | ET | CT | RT | CTRL |
|---|---|---|---|---|
| Age (years) | 68 ± 3 | 67 ± 4 | 69 ± 6 | 66 ± 7 |
| Gender (F/M) | 5/5 | 5/5 | 5/5 | 5/5 |
| PA level (h/week) | 1.1 ± 0.5 | 0.3 ± 0.5 | 0.3 ± 0.5 | 1.1 ± 0.9 |
| Comorbidity (n.) | ||||
| Type II diabetes | 1 | 2 | 1 | 1 |
| Pharmacological treatments (n.) | ||||
| Trazodone | 3 | 2 | 3 | 4 |
| Thiazolidinedioe | 2 | 3 | 2 | 2 |
| Etofylline | 1 | 1 | 1 | 1 |
| Captopril | 8 | 8 | 9 | 7 |
| Clortalidone | 9 | 7 | 7 | 5 |
| Metformin | 1 | 1 | 0 | 1 |
| Sulfonylureas | 1 | 1 | 1 | 0 |
ET endurance training, CT circuit training, RT relaxing training, CTRL controls, F female, M male, PA level hours of physical activity executed by the participants per week
Experimental design and training protocols
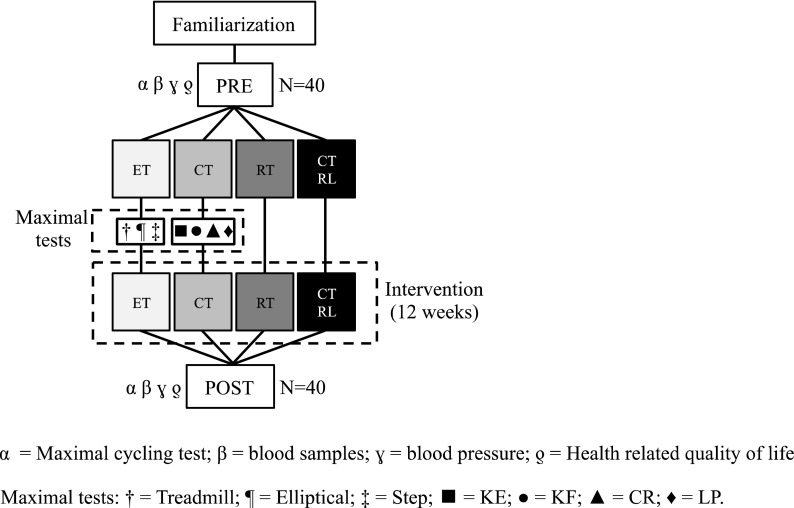
The experimental design, with the proposed interventions, is represented in Fig. 1. All volunteers participated to a series of sessions to familiarize with the exercise protocols and interventions. During the last familiarization session, blood pressure was measured and fasting blood samples were taken. On a different day, participants performed a graded maximal cycle exercise test to assess with indirect calorimetry. After the baseline evaluations (PRE), participants were randomly allocated with stratification for gender to four different groups (n = 10, 5 males + 5 females each group): ET, CT, RT, and hypertensive controls (CTRL).
Fig. 1.
Experimental design: After baseline evaluations (PRE), participants were assigned to four different groups. ET group, endurance exercise training on treadmill, elliptical, and stepper ergometers; CT group, short bouts of dynamic exercises on knee extension (KE), knee flexion (KF), calf rise (CR), and leg press (LP) ergometers; RT group, relaxing training program; CTRL group, no intervention
Training sessions for ET, CT, and RT lasted 60 min each, for three times a week. ET group performed endurance exercise training on treadmill, elliptical, and stepper ergometers. A duration of twenty minutes at 70 % of maximal exercise capacity was set for each ergometer and maintained for the entire duration of the ET program. CT group executed short bouts of dynamic exercises on knee extension, knee flexion, calf rise, and leg press ergometers. CT exercises were performed at 1 Hz at 70 % of the maximal mechanical power. The duration of a single bout of CT exercise was 60 s, with 60 s of recovery in between. RT group participated to a relaxing training program characterized by breathing at 5–6 cycles/min and meditation (Hering et al. 2013). CTRL did not undergo a specific intervention; therefore, their data were utilized as control group. Exercise training compliance was evaluated as a percentage of training sessions attended. All the baseline evaluations were then repeated after 12 weeks of intervention (POST).
Exercise modalities
Cycle exercise was performed on an electromagnetically braked cycle ergometer (Bike-Race Technogym SpA, Gambettola, Italy). Treadmill walking exercise was performed on a motorized inclinable treadmill (Run-Race Technogym SpA, Gambettola, Italy). Elliptical exercise was performed on an elliptical ergometer (Sinchro Technogym SpA, Gambettola, Italy). Step exercise was performed on a stepper ergometer (Step-Race Technogym SpA, Gambettola, Italy). KE, KF, CR, and LP exercises were performed on commercially ergometers (Excite line, Technogym SpA, Gambettola, Italy). RT was performed in a soundproof room. This group of participants, remained supine for the entire duration of the relaxation session, and an expert trainer supervised the training session with verbal feedbacks on the breathing frequency.
Measurements and calculations
Breath-by-breath O2 and CO2 expiratory airflow and HR were continuously recorded and digitized (Quark b2, Cosmed, Rome, Italy) during PRE and POST graded maximal cycle exercise tests. During maximum test, pedal rate was maintained at 60 revolutions per minute (±3 %) and work rate was progressively increased (15 W per min) until voluntary exhaustion. Expiratory ventilation and gas exchange parameters were calculated as the average of the last 30 s of any given workload. Intraclass correlation coefficient (ICC) of the above mentioned parameters are ∼0.85 (Duffield et al. 2004). Both oxygen uptake and power output were expressed in watts for delta efficiency calculation, which was considered as the reciprocal of the slope of the linear relationship between power output and oxygen uptake (three data points corresponded to 80, 95, and 110 W; Poole et al. 1992).
Maximal exercise capacity was determined for ET and CT ergometers with standard graded maximal tests as follows: on the treadmill both speed and slope were progressively increased until voluntary exhaustion (Bruce et al. 1973); on both elliptical and stepper ergometers, work rate was progressively increased (15 W per min), with a cadence at 60 per minute, until voluntary exhaustion. KE, KF, CR, and LP ergometers were instrumented with a commercially available electronic system (TGS-power control, Technogym SpA, Gambettola, Italy). Range of motion, velocity, and power output were continuously monitored and utilized as feedback for participants. Maximal exercise capacity for KE, KF, CR, and LP was assessed with a graded increased workloads protocol, with 1-min steps at a frequency of 60 contractions per min, until voluntary exhaustion. The 70 % of the maximal workload achieved during these evaluations was utilized for ET and CT interventions. HR monitors (Polar RS400) were utilized to measure HR response during the interventions. HR response was assessed also in CTRL while sitting on a chair for a period of time similar to the duration of the intervention. HR reserve (HRR) was calculated as the difference between exercise and resting HR, divided by the difference of age-predicted maximal and resting HRs.
Blood analyses
A fasted venous blood sample was collected from a forearm vein and transported to the laboratory. The serum samples were separated with centrifugation (1,300×g for 15 min, at room temperature). Glucose, high-, and low-density lipoprotein were measured on a Cobas c501 (Roche Diagnostics GmbH, Mannheim, Germany), using proprietary reagents. The standard biological variability of glucose and cholesterol are ±2.3 (mg/dl) and ±5.6 (mg/dl), respectively (Ricos et al. 1999).
Blood pressure
Two different physicians measured blood pressure with standard auscultatory and mercury sphygmomanometer technique at about the same time of the day to minimize the effect of circadian rhythm on the measurement. Both operators repeated the ambulatory blood pressure evaluation 2 times in blind, both before and after intervention. Data reported in the text report the average of the four evaluations.
Health-related quality of life
The Italian version of the SF-36 health survey (Apolone et al. 1998) was administrated before and after the interventions. Briefly, the first four items of the SF-36: physical functioning, role-physical, bodily pain, general health were assessed and categorized in the physical component of the SF-36. Similarly, the remnants 4 items: vitality, social functioning, role-emotional, and mental health were recorded and summarized in the mental component of the SF-36. The items scores were than calculated with the computer-based tool (http://www.sf-36.org; SF-36® Health Survey Health Assessment Lab, Medical Outcomes Trust, and QualityMetric Incorporated).
Statistical analysis
Raw data were analyzed using a statistical software package (IBM SPSS Statistics v. 19, Armonk, NY, USA). To check the normal distribution of the sampling, a Shapiro–Wilk test was applied. A two-way (time and group) ANOVA for repeated measures with a Bonferroni correction test for multiple comparisons was applied on each variable before and after the interventions to assess the effects of training, and the interaction between the two factors. The location of possible differences was assessed by a Holm–Sidak post hoc test. The level of significance was set at α < 0.05. The magnitude of the changes was determined using partial eta squared (η2p) statistics when appropriate (Cohen 1988). η2p was classified as trivial for values <0.2, as small when between 0.2 and 0.6, as moderate between 0.6 and 1.2, as large between 1.2 and 2.0, and very large when >2.0 (Rhea 2004). Given the key role of the variable, ICC and the standard error of measurements calculation as a percentage (SEM%) assessed reliability of the blood pressure measurements. ICC values were consider as very high if >0.90, high if between 0.70 and 0.89 and moderate if between 0.50 and 0.69. Unless otherwise stated, the results are expressed as mean ± standard error (SE).
Results
The participants’ characteristics are summarized in Table 1. No difference was observed between ET, CT, RT and CTRL concerning anthropometrics, comorbidity, and pharmacological treatments (p = 0.8).
Intervention compliance
The small-group approach to the interventions with the supervision of expert operators resulted in 92 ± 3 % compliance for ET, 97 ± 2 % for CT, and 95 ± 4 % for RT, without statistical difference between groups (p = 0.8).
HR response during ET, CT, and RT interventions
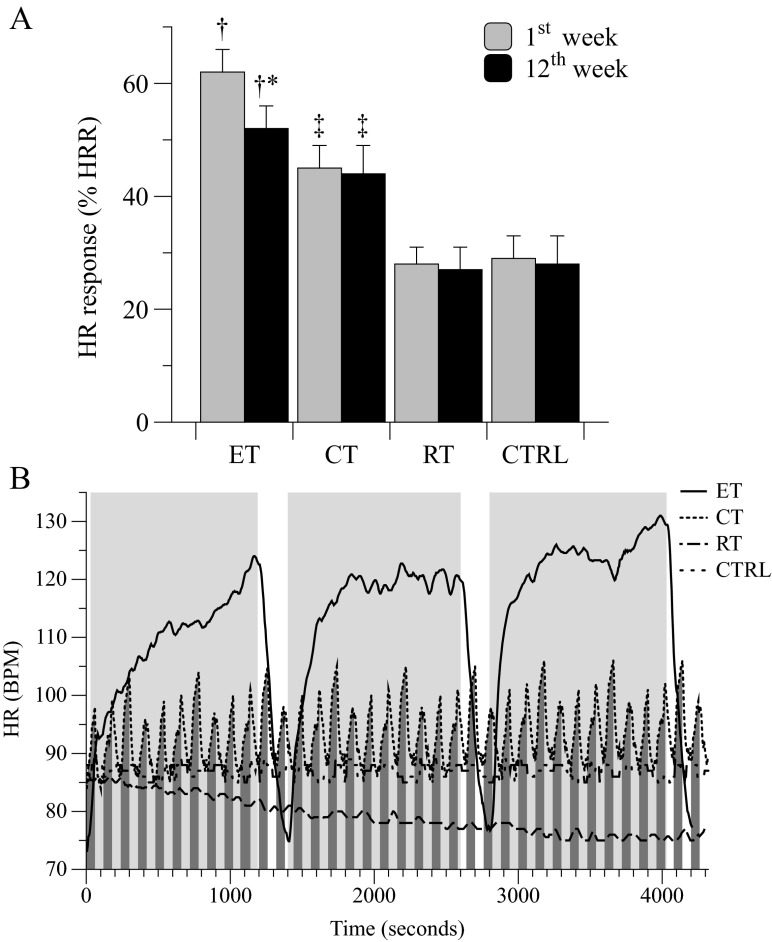
HR response, recorded during a single session in the 1st week of the ET exercises, was 21 % higher respect to CT. Similarly, HR was significantly higher in CT group respect to RT by ∼22 % (Fig. 2; panel A). Despite the exercise-intensity adopted for both ET and CT was 70 % of maximal exercise capacity, the lowest HR was attained during CT due to the short intermittent exercise modality (1 min of exercise followed by 1 min of recovery; Fig. 2; panel B) essential to the premise of the current experimental design. Interestingly, HR recorded during a session of the 12th week of intervention was significantly lower compared to the 1st week only during the ET exercise, while during CT and RT it did not change (Fig. 2; panel A). Data from CTRLs are also provided.
Fig. 2.
Heart rate response: Average heart rate (HR) response, as a percentage of HR reserve (HRR, panel A), in the four groups during interventions at the beginning (1st week) and at the end (12th week) of treatments. Panel B represents the HR response during an intervention session in representative participants of the four groups. The light and dark gray areas represent the exercise time during ET and CT, respectively. * = in-group P < 0.05; † = among groups P < 0.05; ‡ P < 0.05 vs RT and CTRL
Resting HR and blood pressure
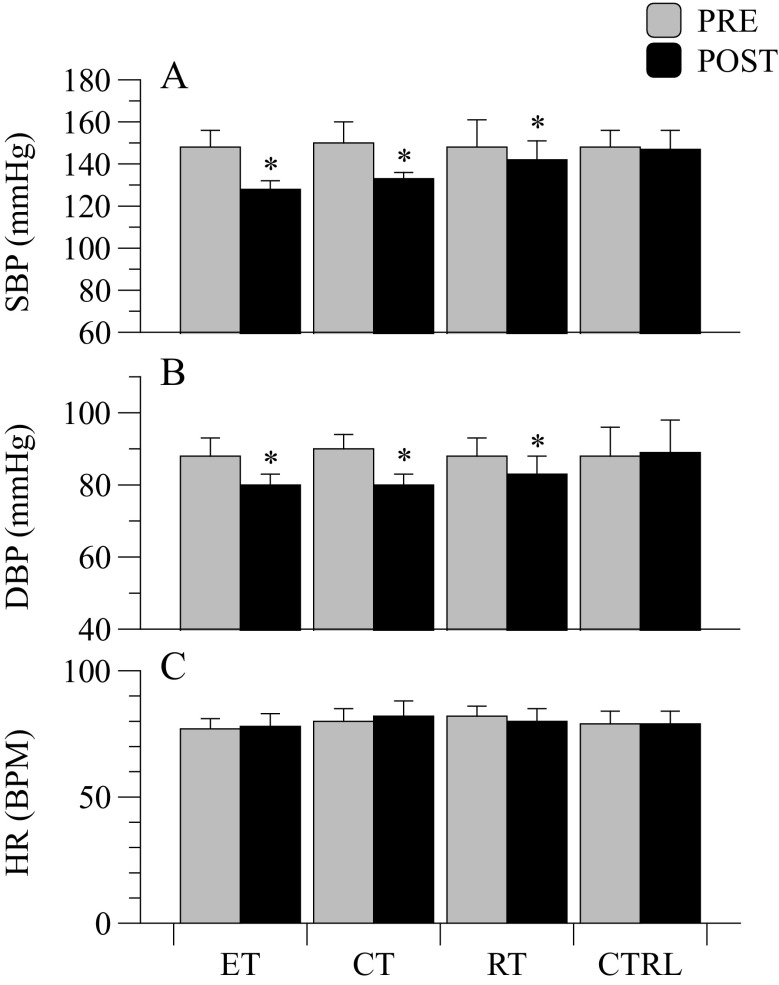
Prior to training, no significant difference in HR, SBP, and DBP were found among ET, CT, RT, and CTRL groups (Fig. 3; panels A, B, C). The ICC determined in this pool of sampling was 0.83 and 0.85 for SBP and DBP, respectively. SEM% was 3.4 and 2.3 % for SBP and DBP, respectively. As a consequence of ET, CT, and RT training, the hypertensive subjects exhibited a significant decrease in SBP (p < 0.001; η2p = 0.867) and DBP (p < 0.001; η2p = 0.821). Significant interactions for SBP and DBP were retrieved (p = 0.001 and η2p = 0.840; p = 0.012 and η2p = 0.672 for SBP and DBP, respectively). Specifically, SBP decreased by 19 ± 4 mmHg, 17 ± 5 mmHg, and 10 ± 3 mmHg in ET, CT, and RT, respectively. The drop in SBP after intervention was similar in ET and CT, but was greater in ET with respect to RT (p < 0.001; η2p = 0.847), as well as in CT with respect to RT (p = 0.006; η2p = 0.847). DBP decreased by 8 ± 2, 10 ± 3, and 7 ± 3 mmHg in ET, CT, and RT respectively. Only the CT group exhibited a greater drop in DBP with respect to RT (p = 0.013; η2p = 0.520). Resting HR didn’t change after the interventions (p = 0.453; η2p = 0.064; Fig. 3; panel C).
Fig. 3.
Cardiovascular variables at rest: Average resting systolic (SBP) and diastolic (DBP) blood pressures and heart rate (HR) in the four groups before and after interventions. * = in-group P < 0.05
Blood analyses and health-related quality of life
At baseline, glucose and cholesterol were not different in ET, CT, RT, and CTRL groups, (p = 0.298; η2p = 0.261; Table 2). The effect of CT was more pronounced in the reduction of glucose (−26 mg/dl; p < 0.001; η2p = 0.922), while ET training, ameliorated both HDL (+9 mg/dl; p = 0.04; η2p = 0.458) and LDL (−14 mg/dl; p = 0.03; η2p = 0.594). Mental component of the SF-36 survey for health-related quality of life was increased for ET, CT, and RT (p < 0.001; η2p = 0.987); however, both ET and CT exhibited a larger improvement compared to RT (p = 0.046; η2p = 0.707).
Table 2.
Blood analyses and health-related
| ET | CT | RT | CTRL | |||||
|---|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | PRE | POST | PRE | POST | |
| Stature (m) | 1.72 ± 0.05 | 1.72 ± 0.05 | 1.75 ± 0.06 | 1.75 ± 0.06 | 1.73 ± 0.05 | 1.73 ± 0.05 | 1.73 ± 0.06 | 1.73 ± 0.06 |
| BMI (kg/m2) | 27 ± 4 | 27 ± 5 | 27 ± 3 | 27 ± 4 | 26 ± 5 | 26 ± 4 | 27 ± 6 | 27 ± 6 |
| Wcirc (cm) | 95 ± 9 | 94 ± 7 | 95 ± 8 | 94 ± 5 | 96 ± 9 | 96 ± 8 | 98 ± 10 | 98 ± 11 |
| Hcirc (cm) | 100 ± 9 | 100 ± 8 | 102 ± 8 | 101 ± 9 | 99 ± 8 | 99 ± 9 | 103 ± 11 | 104 ± 12 |
| Glucose (mg/dl) | 108 ± 11 | 100 ± 6 | 112 ± 7 | 86 ± 4 *† | 104 ± 13 | 100 ± 13 | 101 ± 9 | 101 ± 7 |
| HDL (mg/dl) | 57 ± 9 | 66 ± 9 *† | 55 ± 14 | 58 ± 22 | 64 ± 12 | 59 ± 20 | 59 ± 9 | 56 ± 9 |
| LDL (mg/dl) | 140 ± 33 | 126 ± 13*† | 143 ± 30 | 130 ± 28 | 153 ± 35 | 151 ± 33 | 141 ± 39 | 138 ± 35 |
| Health related quality of life | ||||||||
| SF-36phys (0–100) | 33 ± 4 | 49 ± 4 * | 32 ± 4 | 48 ± 5 * | 34 ± 3 | 36 ± 5 | 37 ± 11 | 37 ± 12 |
| SF-36ment (0–100) | 29 ± 2 | 54 ± 3 * | 29 ± 3 | 53 ± 5 * | 29 ± 3 | 50 ± 4 * | 28 ± 8 | 28 ± 9 |
ET endurance training, CT circuit training, RT relaxing training, CTRL controls, BMI body mass index, W circ waist circumference, H circ hip circumference, HDL high-density lipoprotein, LDL low-density lipoprotein, SF-36phys physical component of health related quality of life, SF-36ment mental component of health related quality of life
* = In-group P < 0.05; † = among groups P < 0.05
All groups demonstrated a significant increase in the physical component of SF-36 (p < 0.001; η2p = 0.970, with greater increase in ET and CT respect to the RT (p = 0.002; η2p = 0.830.
Maximal cycle exercise pre- and post- ET, CT, RT, and CTRL:
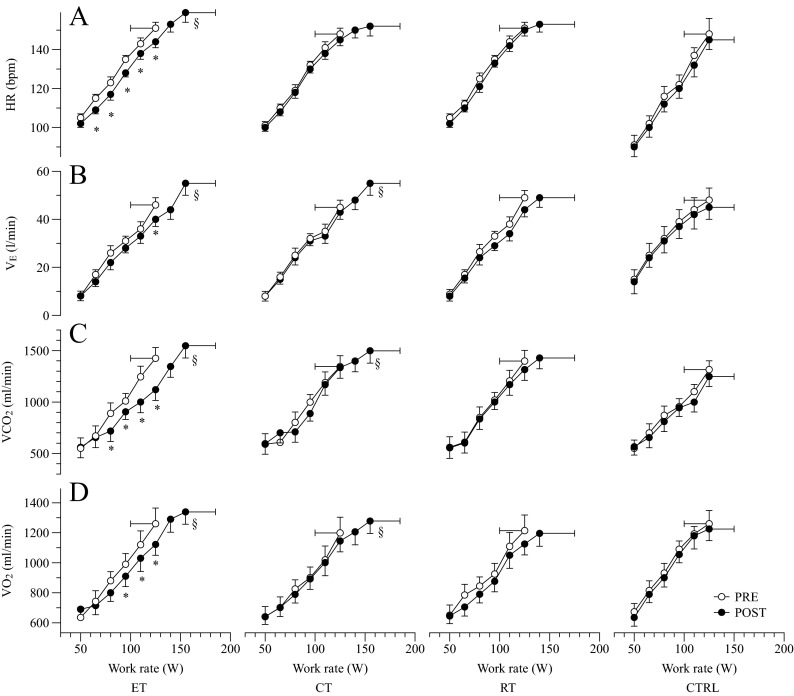
Maximum work rate during cycle exercise increased significantly after the 12 weeks of intervention by ∼30 W for both ET and CT, and by ∼15 W for RT. No change was observed for the CTRL group. Pre-training, cycle was 1,260 ± 104, 1,199 ± 105, 1,214 ± 111, and 1,189 ± 131 ml/min for ET, CT, RT, and CTRL respectively. Post-training cycle was significantly increased in both ET (+8 %; p < 0.001; η2p = 0.712) and CT (+7 %; p < 0.001; η2p = 0.707), while in RT and CTRL was similar to pre-training values (Fig. 4; panel D). Pre-training attained during maximal cycling exercise was similar for all the 4 groups ∼1390 ml/min, however after the intervention peak of was increased by (∼9 %; p = 0.003; η2p = 0.640) in ET, (∼11 %; p = 0.003; η2p = 0.715) in CT, ∼2 % in RT, and ∼1 % in CTRL (Fig. 4; panel C). Pre-training, peak in was not different between groups ∼47 l/min, but similar to the results, was significantly increased in ET (p < 0.001; η2p = 0.972) and CT (p = 0.001; η2p = 0.848) after the interventions (Fig. 4; panel B). Pre-training HR peak reached during cycle exercise was similar ∼150 BPM in the four groups. However, post-training maximum HR was significantly augmented only in ET (∼7 %; p = 0.003; η2p = 0.762) while CT ∼3 %, RT ∼2 %, and CTRL ∼2 % did not changed (Fig. 4; panel A). Significant interactions in (p = 0.006; η2p = 0.723), (p < 0.001; η2p = 0.948), and HR peak (p = 0.004; η2p = 0.749) were found.
Fig. 4.
Cardiorespiratory response to exercise: average heart rate (HR), expiratory ventilation , CO2 production , and oxygen uptake during cycle incremental ramp exercise in the four groups, before and after interventions. * = in-group P < 0.05; § = in-group P < 0.05 at maximal exercise
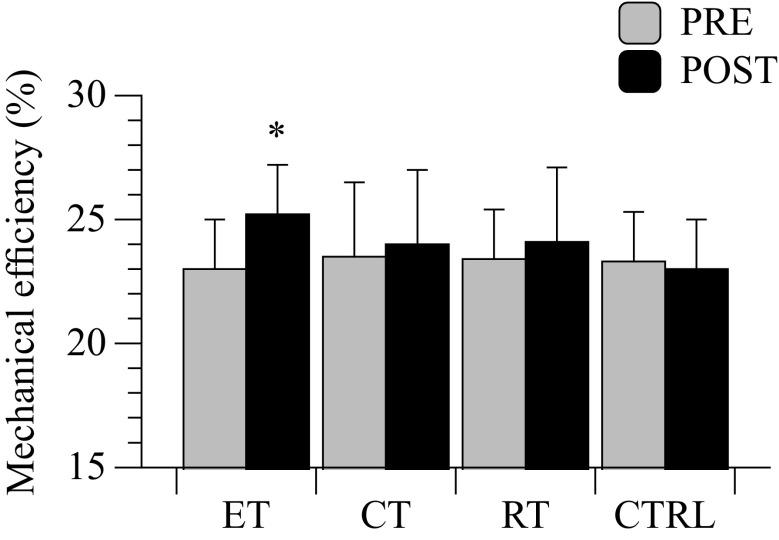
Mechanical efficiency
Twelve weeks of ET resulted in an attenuated oxygen uptake recorded at given submaximal work rates (80–95–110 W) of the maximal cycle exercise. This outcome indicates a significant increase in mechanical efficiency that was obtained only by the ET group (p < 0.001; η2p = 0.857; Fig. 4, panel D; Fig. 5). A significant interaction was found (p = 0.001; η2p = 0.815). Interestingly, patients in ET group exhibited a similar trend in the reduction of HR at submaximal work rates (Fig. 4; panel A), indicating an increased cardiac reserve induced by the ET intervention.
Fig. 5.
Mechanical efficiency: Δ mechanical efficiency in the four groups, calculated on the first part of the cycle ramp exercise before and after interventions. * = in-group P < 0.05
Discussion
With the intent to evaluate the effectiveness of different exercise-based treatments on the modification of CVDs risk factors, exercise capacity, and quality of life of old hypertensive individuals, we investigated the effects of three different interventional strategies (ET, both central and peripheral stimulation; CT, only peripheral stimulation; and RT, no central or peripheral hemodynamic involvement) in elderly patients with grade 1 of HYP. In agreement with our hypothesis, resting systolic and diastolic blood pressures were significantly reduced in all groups. This expected positive outcome was accompanied by different achievements in blood glucose and cholesterol levels, maximum aerobic power, mechanical efficiency and health-related quality of life scores. The reduction in blood pressure in ET was accompanied by a concurrent decrease in blood cholesterol levels and an increase in , mechanical efficiency and quality of life scores. Similarly, in CT, blood pressure amelioration was accompanied by a decrease in blood glucose levels and an increase in and quality of life scores. On the contrary, in RT the lower systolic and diastolic blood pressure went along only with an improvement in the mental component of quality of life.
Exercise-based treatments and CVDs risk factors
Despite the three different approaches were effective in the reduction of HYP, other CVDs risk factors were affected differently by ET, CT, and RT. Specifically, the drop in SBP and DBP was significant, but with different extent in the ET, CT, and RT. In fact, both ET and RT demonstrated a greater reduction of HYP in comparison to the subjects that performed RT. Moreover, HDL and LDL, two important CVDs risk factors, were significantly ameliorated after the ET treatment, while the effect on cholesterol was not significant for CT, and RT. This result is in agreement with the majority of the literature, and it is likely caused by the predominant utilization of the free-fat acids derived from cholesterol during the ET (Braz et al. 2012).
Interestingly, while ET and RT exhibited a positive, but not significant, trend in the reduction of glucose, the effect of CT was more pronounced, even though we cannot completely rule out possible effects of medications in this group with two individuals with type II diabetes instead of one, as in the other groups. These positive results on glucose reduction are in the range of outcomes usually retrieved in aerobic and resistance training (Short et al. 2003). Noticeably, this additional positive outcome reinforces the relationship between the positive effects of physical exercise and cardiovascular health (Guimaraes et al. 2010; Sousa et al. 2013). Collectively, it appears that non-pharmacological interventions based upon active exercise training, ET and CT, were both successful in the amelioration of several CVDs risk factors, while RT was less effective.
Exercise-based treatments and quality of life
Quality of life is a relevant aspect of health in old patients with HYP. It is well established that physical exercise can positively change these psychological and health-condition factors in this population (Tolonen et al. 2013). Our data on the mental component of the SF-36 confirm and advance these results, underlining that also a RT approach can improve the psychological component of quality of life. However, the results of physical component of the SF-36 survey indicate that the patients’ perception of his/her health status increased only after a period of active exercise training (ET or CT). This contrast can be explained by the additional, and assessed in the current study, positive health-related improvements (blood pressure, glucose and cholesterol levels, and exercise capacity) obtained by ET and CT interventions. Therefore, it appears that these additional health-related gains obtained by the employment of active exercise, may predispose old individuals with HYP to a better perception of their quality of life. Again, the overall better responses on the CVDs risk factors together with the improved quality of life observed in our study, indicate that the best choices in terms of non-pharmacological treatment for HYP reduction are ET and CT.
Exercise-based treatments and maximal exercise capacity
Maximal aerobic capacity, defined by , is a strong predictor of cardiovascular health and independence in older adults (Paterson et al. 2004) and hypertensives (Totsikas et al. 2011). Thus, the investigation of the effectiveness of non-pharmacological treatment has clear practical significance in terms of identifying the means by which the capacity for an independent lifestyle can be maintained (Mancia et al. 2013a). Our data indicate that both ET and CT enhanced significantly the maximal exercise capacity assessed during cycle maximal test. According to the maximal work rate, increased significantly in both ET (+8 %) and CT (+7 %). Moreover, the lack of difference in maximal exercise capacity exhibited by the participants assigned to RT intervention suggests that this non-pharmacological approach for HYP reduction is not effective on the enhancement of . Therefore, this additional result in favor of the ET and CT treatments suggests that the adoption of an active lifestyle characterized by the practicing of dynamic exercise has a remarkable effect not only on blood pressure, but also in maximal exercise capacity, and possibly in the independence of old hypertensives.
Another important outcome exhibited by the ET group, was the significant increase in the HR recorded at maximum exercise. It is important to note that this positive gain of ∼7 % was not obtained by the other groups, implying that only after an ET intervention a positive effect on the heart hemodynamics could be retrieved. Several studies emphasized the importance of cardiovascular stimuli, such as ET, that increase maximal cardiac output and HR, by which maximal exercise capacity can be enhanced (Carrick-Ranson et al. 2014). However, our data suggest that even with CT, characterized by a very limited cardiovascular stimulus, the was positively increased. This equal response in the maximal aerobic capacity obtained by the employment of these different approaches suggests that different factors affected this result. Indeed, the positive effect of ET on was likely caused by both central and peripheral adaptation, such as a better heart hemodynamics (Rodrigues et al. 2012), an increased skeletal muscle capillarization (Hansen et al. 2010), a higher nitric oxide (NO) bioavailability (Blanco-Rivero et al. 2013), and an increased mitochondrial density (Pesta et al. 2011). On the contrary, the increased maximal exercise capacity demonstrated after CT was primarily induced by peripheral adaptations (Gibala et al. 2012; Hood et al. 2011), predominantly related to a preserved response of mitochondrial function to this short-intermittent training.
Exercise-based treatments and mechanical efficiency
The is certainly one of the best predictors of independence. However, the metabolic demands obtained during the everyday tasks, such as walking, stairs climbing, and housekeeping, are only a fragment of the maximal aerobic capacity measured during a maximal test (Astrand 2003). On the contrary, mechanical efficiency at submaximal intensity is more representative of the work economy by which the activities of daily life can be executed, and, in turn, has a clear consequence on the independence and quality of life of this old population (Venturelli et al. 2012a, 2013; b). In this scenario, it has been demonstrated that mechanical efficiency is significantly reduced in sedentary patients with pulmonary and heart dysfunctions (Perrault 2006; Richardson et al. 2004; Riescher et al. 2004). Moreover, the recent literature reports that non-pharmacological interventions, based upon high intensity exercise, are effective for the enhancement of mechanical efficiency (Hoff et al. 2007; Karlsen et al. 2009). Interestingly, our data revealed that mechanical efficiency was significantly ameliorated only after ET (Fig. 5). Conversely, mechanical efficiency after CT and RT treatments was unchanged, suggesting that in old hypertensive individuals the stimulation of both central and peripheral factors is required to obtained a significant gain in mechanical efficiency. This positive finding was likely the consequence of: (i) an increased heart economy, exhibited by a reduction in cardiac output and HR, with a concomitant increase in stroke volume during exercise at given submaximal work rates; and (ii) a peripheral adaptation to a slower skeletal muscle fiber phenotype (Gibbs et al. 1972; Hunter et al. 2001).
Overall physiological considerations
Despite direct measurements were not actually performed, a decrease in cardiac output and total peripheral resistance at rest and during exercise generally accompany the ET-induced reduction in arterial pressure observed in the present study (Brito Ade et al. 2014). Other factors associated with these changes are likely a decrease in the peripheral vascular resistance, mediated by a reduction in sympathetic neural drive, and an increase in the bioavailability of local vasodilator factors, (i.e., NO) (Seals et al. 2011). Moreover, structural adaptations in arterial stiffness at the peripheral level, increases in lumen diameter of conduit arteries, and adjustments in vascular bed (i.e., increased number of vessels) likely elevate the total vessels cross-sectional area and contribute to the reduction in blood pressure after ET (Pescatello et al. 2004). Additionally, ET is a strong cardiovascular stimulus that affects positively also heart hemodynamics, therefore these central effects of ET likely contributed to the reduction in HR during exercise (see Fig. 2, panel A, and Fig. 4, panel A). The significant enhancement in maximum HR (∼7 %) after ET may largely contribute to the improved (∼8 %). Therefore, the similar extent in the ameliorations in maximum HR and pulmonary may suggest an important role of a bettered central hemodynamics in the improvement of maximum aerobic capacity. Further studies involving also cardiac output measurements may provide additional support to this explanation. Certainly, as reported in previous other studies (Beck et al., 2013; Mortensen et al., 2013), we cannot neglect that the effects of ET on can be ascribed to both central and peripheral components of oxygen uptake. Unfortunately, the lack of peripheral measurements in the present study cannot provide further insights on this aspect.
Noticeably, the physiological mechanisms that induced a reduction in blood pressure and increased after CT were different to those involved in ET in terms of central hemodynamics stimulation. Certainly, repeated one-minute bouts of dynamic exercises produce limited HR and cardiac output responses because of the slower onset kinetics of central hemodynamic (Scheuermann et al. 2002), with high stimulation of the peripheral circulation because of the multiple exercise onsets that induce cyclic hyperemia (Clifford 2007). Consequently, peripheral adaptations in the vasculature, increased levels of NO bioavailability, and modifications of the skeletal muscle phenotype were likely involved in these responses to CT intervention.
On the other hand, slow breathing at ∼6 cycles/min increases baroreflex sensitivity, reduces muscle sympathetic nerve activity and chemoreflex activation (Goso et al. 2001; Spicuzza et al. 2000). Thus, the significant reduction in blood pressure observed after the RT intervention was likely due to these mechanisms.
Study limitations
A limitation of the current study was the relatively small sample size, which may have influenced the differences induced by the training adopted in the old hypertensive participants. However, due to the complexity of the study and the limited availability of the participants eligible for the present investigation, the sample size was restricted to the actual dimension. Additionally, to better describe the significance of the outcomes in this relatively small sample size of patients, the results has been expressed comprehensively of the effect size calculation (Bonferroni correction test for multiple comparisons). Further limitations were the mixed gender of the sample, and the potential effects of comorbidities, such as type II diabetes. However, with respect to the latter issues, the old male and female participants, as well as individuals with type II diabetes were normally distributed in the four groups.
Conclusion
The choice of exercise-based treatments for the reduction of CVDs risk factors has to take into account not only the direct effects on cardiovascular health, but also other accessory outcomes that can affect the independence and quality of life of old hypertensive individuals. Moreover, personal psychological and economical barriers need to be accounted for the efficacy of the treatment. Therefore, it appears that the employment of an active lifestyle characterized by the execution of ET is the best choice to reduce CVDs risk factors, because the amelioration of HYP is accompanied by decreases in blood glucose and cholesterol levels, increases in maximal exercise capacity, mechanical efficiency, and quality of life. Other workouts characterized by short intermittent dynamic exercises are equally effective in the reduction of HYP and improvement of . However, CT appears less effective in the enhancement of mechanical efficiency. Alternative trainings, based on breath control and meditation, are similarly successful in the reduction of HYP. Despite the amelioration in blood pressure was less extended, the additional benefits on quality of life induced by RT can motivate patients for this kind of practice, and would be an attractive alternative for people unable or reluctant to carry out ET or CT. Further studies on long-term effects of the present exercise-based interventions duration may be required to enhance the knowledge on this matter.
Acknowledgments
The authors greatly appreciate the time and effort of the patients that participated to this study. We wish to thank Stefano Zucca and Alessio Sollima for their valuable assistance with patients’ coordination, during the evaluations and exercise interventions.
References
- Apolone G, Mosconi P. The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol. 1998;51(11):1025–1036. doi: 10.1016/S0895-4356(98)00094-8. [DOI] [PubMed] [Google Scholar]
- Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, Ferdinand KC, Forciea MA, Frishman WH, Jaigobin C, Kostis JB, Mancia G, Oparil S, Ortiz E, Reisin E, Rich MW, Schocken DD, Weber MA, Wesley DJ, Harrington RA, Force AT. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2011;123(21):2434–2506. doi: 10.1161/CIR.0b013e31821daaf6. [DOI] [PubMed] [Google Scholar]
- Astrand P-O. Textbook of work physiology: physiological bases of exercise. Champaign: Human Kinetics; 2003. [Google Scholar]
- Bartlett JD, Close GL, MacLaren DP, Gregson W, Drust B, Morton JP. High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: implications for exercise adherence. J Sports Sci. 2011;29(6):547–553. doi: 10.1080/02640414.2010.545427. [DOI] [PubMed] [Google Scholar]
- Blanco-Rivero J, Roque FR, Sastre E, Caracuel L, Couto GK, Avendano MS, Paula SM, Rossoni LV, Salaices M, Balfagon G. Aerobic exercise training increases neuronal nitric oxide release and bioavailability and decreases noradrenaline release in mesenteric artery from spontaneously hypertensive rats. J Hypertens. 2013;31(5):916–926. doi: 10.1097/HJH.0b013e32835f749c. [DOI] [PubMed] [Google Scholar]
- Braz NF, Carneiro MV, Oliveira-Ferreira F, Arrieiro AN, Amorim FT, Lima MM, Avelar NC, Lacerda AC, Peixoto MF. Influence of aerobic training on cardiovascular and metabolic parameters in ederly hypertensive women. Int J Prevent Med. 2012;3(9):652–659. [PMC free article] [PubMed] [Google Scholar]
- Brito Ade F, de Oliveira CV, Santos Mdo S, Santos Ada C. High-intensity exercise promotes postexercise hypotension greater than moderate intensity in elderly hypertensive individuals. Clin Physiol Funct Imaging. 2014;34(2):126–132. doi: 10.1111/cpf.12074. [DOI] [PubMed] [Google Scholar]
- Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85(4):546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- Cadore EL, Izquierdo M. Exercise interventions in polypathological aging patients that coexist with diabetes mellitus: improving functional status and quality of life. Age. 2015;37(3):9800. doi: 10.1007/s11357-015-9800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrick-Ranson GC, Hastings JL, Bhella PS, Fujimoto N, Shibata S, Palmer MD, Boyd K, Livingston S, Dijk E, Levine BD. The effect of lifelong exercise dose on cardiovascular function during exercise. J Appl Physiol. 2014 doi: 10.1152/japplphysiol.00342.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford PS. Skeletal muscle vasodilatation at the onset of exercise. J Physiol. 2007;583(Pt 3):825–833. doi: 10.1113/jphysiol.2007.135673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988) Statistical power analysis for the behavioral sciences. Lawrence Erlbaum associates
- Duffield R, Dawson B, Pinnington HC, Wong P. Accuracy and reliability of a Cosmed K4b2 portable gas analysis system. J Sci Med Sport. 2004;7(1):11–22. doi: 10.1016/S1440-2440(04)80039-2. [DOI] [PubMed] [Google Scholar]
- Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD, Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol. 2010;55(18):1945–1954. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Reese V, Shabetai R, Wagner PD, Richardson RS. Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: the role of skeletal muscle convective and diffusive oxygen transport. J Am Coll Cardiol. 2011;58(13):1353–1362. doi: 10.1016/j.jacc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590(Pt 5):1077–1084. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs CL, Gibson WR. Energy production of rat soleus muscle. Am J Physiol. 1972;223(4):864–871. doi: 10.1152/ajplegacy.1972.223.4.864. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C and Stroke Statistics S Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goso Y, Asanoi H, Ishise H, Kameyama T, Hirai T, Nozawa T, Takashima S, Umeno K, Inoue H. Respiratory modulation of muscle sympathetic nerve activity in patients with chronic heart failure. Circulation. 2001;104(4):418–423. doi: 10.1161/hc2901.093111. [DOI] [PubMed] [Google Scholar]
- Guimaraes GV, Ciolac EG, Carvalho VO, D’Avila VM, Bortolotto LA, Bocchi EA. Effects of continuous vs. interval exercise training on blood pressure and arterial stiffness in treated hypertension. Hypertens Res. 2010;33(6):627–632. doi: 10.1038/hr.2010.42. [DOI] [PubMed] [Google Scholar]
- Hansen AH, Nielsen JJ, Saltin B, Hellsten Y. Exercise training normalizes skeletal muscle vascular endothelial growth factor levels in patients with essential hypertension. J Hypertens. 2010;28(6):1176–1185. doi: 10.1097/HJH.0b013e3283379120. [DOI] [PubMed] [Google Scholar]
- Hering D, Kucharska W, Kara T, Somers VK, Parati G, Narkiewicz K. Effects of acute and long-term slow breathing exercise on muscle sympathetic nerve activity in untreated male patients with hypertension. J Hypertens. 2013;31(4):739–746. doi: 10.1097/HJH.0b013e32835eb2cf. [DOI] [PubMed] [Google Scholar]
- Hoff J, Tjonna AE, Steinshamn S, Hoydal M, Richardson RS, Helgerud J. Maximal strength training of the legs in COPD: a therapy for mechanical inefficiency. Med Sci Sports Exerc. 2007;39(2):220–226. doi: 10.1249/01.mss.0000246989.48729.39. [DOI] [PubMed] [Google Scholar]
- Hood MS, Little JP, Tarnopolsky MA, Myslik F, Gibala MJ. Low-volume interval training improves muscle oxidative capacity in sedentary adults. Med Sci Sports Exerc. 2011;43(10):1849–1856. doi: 10.1249/MSS.0b013e3182199834. [DOI] [PubMed] [Google Scholar]
- Hunter GR, Newcomer BR, Larson-Meyer DE, Bamman MM, Weinsier RL. Muscle metabolic economy is inversely related to exercise intensity and type II myofiber distribution. Muscle Nerve. 2001;24(5):654–661. doi: 10.1002/mus.1051. [DOI] [PubMed] [Google Scholar]
- Karlsen T, Helgerud J, Stoylen A, Lauritsen N, Hoff J. Maximal strength training restores walking mechanical efficiency in heart patients. Int J Sports Med. 2009;30(5):337–342. doi: 10.1055/s-0028-1105946. [DOI] [PubMed] [Google Scholar]
- Lamina S. Effects of continuous and interval training programs in the management of hypertension: a randomized controlled trial. J Clin Hypertens. 2010;12(11):841–849. doi: 10.1111/j.1751-7176.2010.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Collaboration PS Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Council ESHS, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, ESCCfP Guidelines. Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Ryden L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34(28):60. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, List of authorsTask Force M 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46(3):454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- Oken BS, Zajdel D, Kishiyama S, Flegal K, Dehen C, Haas M, Kraemer DF, Lawrence J, Leyva J. Randomized, controlled, six-month trial of yoga in healthy seniors: effects on cognition and quality of life. Altern Ther Health Med. 2006;12(1):40–47. [PMC free article] [PubMed] [Google Scholar]
- Pal S, Radavelli-Bagatini S, Ho S. Potential benefits of exercise on blood pressure and vascular function. J Am Soc Hypertens. 2013;7(6):13. doi: 10.1016/j.jash.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Patel C. 12-month follow-up of yoga and bio-feedback in the management of hypertension. Lancet. 1975;7898:62–64. doi: 10.1016/S0140-6736(75)91070-3. [DOI] [PubMed] [Google Scholar]
- Paterson DH, Govindasamy D, Vidmar M, Cunningham DA, Koval JJ. Longitudinal study of determinants of dependence in an elderly population. J Am Geriatr Soc. 2004;52(10):1632–1638. doi: 10.1111/j.1532-5415.2004.52454.x. [DOI] [PubMed] [Google Scholar]
- Perrault H. Efficiency of movement in health and chronic disease. Clin Invest Med. 2006;29(2):117–121. [PubMed] [Google Scholar]
- Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. Exercise and hypertension. Med Sci Sports Exerc. 2004;36(3):533–553. doi: 10.1249/01.MSS.0000115224.88514.3A. [DOI] [PubMed] [Google Scholar]
- Pesta D, Hoppel F, Macek C, Messner H, Faulhaber M, Kobel C, Parson W, Burtscher M, Schocke M, Gnaiger E. Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Reg Integr Comp Physiol. 2011;301(4):R1078–R1087. doi: 10.1152/ajpregu.00285.2011. [DOI] [PubMed] [Google Scholar]
- Poole DC, Gaesser GA, Hogan MC, Knight DR, Wagner PD. Pulmonary and leg VO2 during submaximal exercise: implications for muscular efficiency. J Appl Physiol. 1992;72(2):805–810. doi: 10.1152/jappl.1992.72.2.805. [DOI] [PubMed] [Google Scholar]
- Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37(5):1199–1208. doi: 10.1161/01.HYP.37.5.1199. [DOI] [PubMed] [Google Scholar]
- Rhea MR. Determining the magnitude of treatment effects in strength training research through the use of the effect size. J Strength Cond Res. 2004;18(4):918–920. doi: 10.1519/14403.1. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Leek BT, Gavin TP, Haseler LJ, Mudaliar SR, Henry R, Mathieu-Costello O, Wagner PD. Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak VO2 with small muscle mass exercise. Am J Respir Crit Care Med. 2004;169(1):89–96. doi: 10.1164/rccm.200305-627OC. [DOI] [PubMed] [Google Scholar]
- Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, Minchinela J, Perich C, Simon M. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999;59(7):491–500. doi: 10.1080/00365519950185229. [DOI] [PubMed] [Google Scholar]
- Riescher B, Bourdarias JP, Dubourg O, Jondeau G. Skeletal muscle efficiency in heart failure. Ann Cardiol Angeiol. 2004;53(4):188–192. doi: 10.1016/j.ancard.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Rodrigues B, Jorge L, Mostarda CT, Rosa KT, Medeiros A, Malfitano C, de Souza AL, Jr, Viegas KA, Lacchini S, Curi R, Brum PC, De Angelis K, Irigoyen MC. Aerobic exercise training delays cardiac dysfunction and improves autonomic control of circulation in diabetic rats undergoing myocardial infarction. J Cardiac Fail. 2012;18(9):734–744. doi: 10.1016/j.cardfail.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Romero-Arenas S, Martinez-Pascual M and Alcaraz PE (2013) Impact of resistance circuit training on neuromuscular, cardiorespiratory and body composition adaptations in the elderly. Aging Dis 4(5):256–63. doi: 10.14336/AD.2013.0400256 [DOI] [PMC free article] [PubMed]
- Santaella DF, Araujo EA, Ortega KC, Tinucci T, Mion D, Jr, Negrao CE, de Moraes Forjaz CL. Aftereffects of exercise and relaxation on blood pressure. Clin J Sport Med. 2006;16(4):341–347. doi: 10.1097/00042752-200607000-00010. [DOI] [PubMed] [Google Scholar]
- Scheuermann BW, Bell C, Paterson DH, Barstow TJ, Kowalchuk JM. Oxygen uptake kinetics for moderate exercise are speeded in older humans by prior heavy exercise. J Appl Physiol. 2002;92(2):609–616. doi: 10.1152/japplphysiol.00186.2001. [DOI] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci. 2011;120(9):357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52(8):1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- Sousa N, Mendes R, Abrantes C, Sampaio J, Oliveira J. Long-term effects of aerobic training versus combined aerobic and resistance training in modifying cardiovascular disease risk factors in healthy elderly men. Geriatr Geront Int. 2013;13(4):928–935. doi: 10.1111/ggi.12033. [DOI] [PubMed] [Google Scholar]
- Spicuzza L, Gabutti A, Porta C, Montano N, Bernardi L. Yoga and chemoreflex response to hypoxia and hypercapnia. Lancet. 2000;356(9240):1495–1496. doi: 10.1016/S0140-6736(00)02881-6. [DOI] [PubMed] [Google Scholar]
- Tolonen H, Koponen P, Mindell J, Mannisto S, Kuulasmaa K. European health examination survey--towards a sustainable monitoring system. Eur J Pub Health. 2013 doi: 10.1093/eurpub/ckt107. [DOI] [PubMed] [Google Scholar]
- Totsikas C, Rohm J, Kantartzis K, Thamer C, Rittig K, Machann J, Schick F, Hansel J, Niess A, Fritsche A, Haring HU, Stefan N. Cardiorespiratory fitness determines the reduction in blood pressure and insulin resistance during lifestyle intervention. J Hypertens. 2011;29(6):1220–1227. doi: 10.1097/HJH.0b013e3283469910. [DOI] [PubMed] [Google Scholar]
- Venturelli M, Richardson RS, Ortega J. Skeletal muscle mechanical efficiency does/does not increase with age. J Appl Physiol. 2012 doi: 10.1152/japplphysiol.01438.2012. [DOI] [PubMed] [Google Scholar]
- Venturelli M, Schena F, Richardson RS. The role of exercise capacity in the health and longevity of centenarians. Maturitas. 2012;73(2):115–120. doi: 10.1016/j.maturitas.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturelli M, Schena F, Scarsini R, Muti E, Richardson RS. Limitations to exercise in female centenarians: evidence that muscular efficiency tempers the impact of failing lungs. Age. 2013;35(3):861–870. doi: 10.1007/s11357-011-9379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Haddad A, Su SW, Celler BG, Coutts AJ, Duffield R, Donges CE, Nguyen HT. An equivalent circuit model for onset and offset exercise response. Biomed Eng Online. 2014;13(1):145. doi: 10.1186/1475-925X-13-145. [DOI] [PMC free article] [PubMed] [Google Scholar]