Abstract
The role of the hippocampus in postural control, in particular in maintaining upright stance, has not been fully examined in normal aging. This study aims to examine the association of postural sway with hippocampal volume while maintaining upright stance in healthy older individuals. Seventy healthy individuals (mean age 69.7 ± 3.4 years; 41.4 % women) were recruited in this study based on cross-sectional design. Hippocampal volume (quantified from a three-dimensional T1-weighted MRI using semi-automated software), three center of pressure (COP) motion parameters (sway area, path length of anterior-posterior (AP) and medial-lateral (ML) displacement) while maintaining upright stance (eyes open and closed), and the relative difference between open and closed eye conditions were used as outcome measures. Age, sex, body mass index, lower limb proprioception, distance vision, 15-item geriatric depression scale score, total cranial volume, and white matter abnormalities were used as covariates. The sway area decreased from open to closed eye condition but this variation was non-significant (P = 0.244), whereas path length of AP and ML displacement increased significantly (P < 0.003). Increase in sway area from open to closed eyes was associated with greater hippocampal volume (β −18.21; P = 0.044), and a trend for an association of increase in path length of AP displacement (P = 0.075 for open eyes and P = 0.071 for closed eyes) with greater hippocampal volume was reported. The hippocampus is involved in upright postural control in normal aging, such that an increase in sway area of COP motion from open to closed eyes is associated with greater hippocampal volume in healthy older adults.
Keywords: Posture, Postural sway, Hippocampus, Magnetic resonance imaging, Motor control, Older adults
Introduction
The hippocampus is a small brain region located within the brain’s medial temporal lobe (Sasaki et al. 2014). It has an important role in the consolidation of memory information and in spatial memory as well as navigation (Sasaki et al. 2014). Although the hippocampus initiates processes involving control of motor responses to sensory stimuli, its role in the integration of sensorimotor processes involved in upright postural control has been poorly studied in normal aging (Bast and Feldon 2003; Borel and Alescio-Lautier 2014).
Impaired balance is common in patients with Alzheimer’s disease (AD) and becomes more prevalent with increasing severity of AD, which in part explains the higher risk of falling compared to cognitively healthy individuals (CHI) (Tangen et al. 2014; Leandri et al. 2009; Sasaki et al. 2014). In parallel, the hippocampus is a brain region specifically affected by neurodegenerative lesions of AD leading to its atrophy (Beauchet et al. 2015a, b), suggesting that lower hippocampal volume could be related to impaired upright postural control. Only one study has previously examined the influence of hippocampal volume on control of postural stability while walking in CHI and in patients with mild cognitive impairment (Beauchet et al. 2015a). This study reported no association (Beauchet et al. 2015a). The particular involvement of the hippocampus in static postural control in CHI remains to be determined.
Postural stability depends on the ability to maintain the body’s center of gravity (COG) above a relatively small base of support (Bruijn et al. 2013). Clinically, this control is characterized by upright postural sway in the anterior-posterior (AP) and medial-lateral (ML) directions, which moves randomly within a perimeter of stability during a stable upright position (Bruijn et al. 2013; Borel and Alescio-Lautier 2014). Several physiological sensorimotor subsystems, such as lower limb proprioception, vestibular output, vision, and cognition, contribute to upright postural control (Borel and Alescio-Lautier 2014; Lord et al. 1991). The measurement of static upright postural sway in the AP and ML directions on a firm surface with eyes open and closed is usually used to examine the fidelity of the postural control system, such that an increase in postural sway represents an impaired postural control (Desai et al. 2010). Recently, we reported that episodic memory impairment was associated with a relative decrease in sway area of the center of pressure (COP) (the area defined by the COP excursion across the support surface) from open to closed eyes in non-demented community-dwelling older adults (Beauchet et al. 2015b). This association suggests that a relative decrease in sway area of COP could be a marker of an early impairment of the highest levels of upright postural control. From a neuroanatomical perspective, these findings also suggest that the hippocampus—a key brain area for episodic memory functioning (Sasaki et al. 2014)—may be involved in postural control.
As AD patients present a reduced hippocampal volume (Dubois et al. 2009) as well as poor balance (Tangen et al. 2014; Leandri et al. 2009), and as we have previously reported an association of decreased sway area from open to closed eyes with lower episodic memory performance, we hypothesized that hippocampal volume would be associated with static postural control. More precisely, lower hippocampal volume would be associated with decreased sway area of COP from open to closed eyes in an upright position. This study aims to examine the association of upright postural sway on a firm surface with hippocampal volume in healthy older individuals. Establishing the association between hippocampal volume and upright postural control may provide new insights into the neural basis of postural instability that could influence fall prevention strategies.
Material and methods
Participants
A total of 70 cognitively healthy participants, referred for the evaluation of memory complaints at the memory clinic of Angers University Hospital (France), were recruited between November 2009 and July 2010 in the “Gait and Alzheimer Interactions Tracking” (GAIT) study, which is an ongoing study based on a cross-sectional design. The study procedures have been described in detail elsewhere (Beauchet et al. 2015a, b). The eligibility criteria were 65 years of age and over, ambulatory, with an adequate understanding of French, and no acute medical illness in the past month. For this study, exclusion criteria were neurological and psychiatric diseases (i.e., dementia or mild cognitive impairment (MCI), moderate and severe medical conditions affecting walking and posture and no standing postural assessment. The following standardized tests were used to probe several aspects of cognitive function: mini-mental state examination (MMSE) (Folstein et al. 1975), frontal assessment battery (FAB) (Dubois et al. 2000), French version of the free and cued selective reminding test—total recall (FCSRT-TR) (Van der Linden et al. 2004), the direct (i.e., forward) digit span (Wechsler 1987), trail making test (TMT) parts A and B (Brown et al. 1958), Stroop test (Stroop 1935), and instrumental activities of daily living scale (IADL) (Pérès et al. 2006). The diagnosis of MCI was established following the Winblad consensus criteria during multidisciplinary meetings involving geriatricians, neurologists, and neuropsychologists of Angers University Memory Clinic (Winblad et al. 2004). Diagnoses of dementia were assigned according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (1994) at consensus diagnostic case conferences. Participants in the study gave their written informed consent and the ethics committee of the Angers University Hospital (France) approved the GAIT study.
Clinical assessment
Age and sex were recorded. Binocular distance vision was measured at 5 m with a standard Monoyer letter chart and scored from 0 (i.e., worst performance) to 10 (i.e., best performance) (Lord et al. 1991). Vision was assessed with corrective lenses if needed. Lower limb proprioception was evaluated with a graduated tuning fork placed on the tibial tuberosity measuring vibration threshold (Beauchet et al. 2011). The mean value obtained for the left and right sides ranged between 0 (i.e., worst performance) and 8 (i.e., best performance) and was used in the present data analysis. The 15-item Geriatric Depression Scale (GDS) was used to screen for depressive symptoms (Launay et al. 2013).
Standing postural sway assessment
Upright postural sway was measured on a firm surface using a force platform (101 × 101 cm; BioRescue, Dune®, France) (Beauchet et al. 2011). This instrument uses vertically oriented force transducers to determine the instantaneous fluctuations of the total body center of mass (COM) as represented by the center of pressure (COP). The participants were asked to maintain barefoot standing position with eyes opened or closed with each foot positioned against a foot frame on a platform plate that maintained the distance between the medial sides of the heel at 8.4 cm with an external rotation angle of 9°. Participants were instructed to look straight ahead, with arms kept by the side of the body, for 30 s. From the balance system software, three types of COP motion parameters were used as outcome measures: the mean sway area (i.e., the total area of the AP and ML COP displacement, expressed in mm2) and the path length of COP displacement in AP and LM directions (expressed in mm). In addition, we computed the change (i.e., the relative difference) between the eyes-open and eyes-closed conditions using the formula: (COP parameter eyes-open – COP parameter eyes-closed / [(COP parameter eyes-open + COP parameter eyes-closed) / 2] × 100.
Hippocampal volume
Brain imaging was performed with a 1.5-Tesla MRI scanner (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany) using a standard MRI protocol including (MP-RAGE) axial (acquisition matrix = 256 × 256 × 144, FOV = 240 mm × 240 mm × 187 mm, TE/TR/TI = 4.07 ms/2170 ms/1100 ms), and fluid-attenuated inversion recovery (FLAIR) axial images (acquisition matrix = 256 × 192, FOV = 240 mm × 180mm, pixel size: 0.9 × 0.9 × 5.5 mm; slice thickness = 5 mm, slice gap = 0.5 mm, 30 slices, TE/TR/TI = 122 ms/9000 ms/2500 ms) (Dubois et al. 2009).
The volumetric 3D T1-weighted images were segmented using the FreeSurfer software package (version 5.1.0; 33) to calculate the hippocampal volume. The procedure has been previously described in detail (Beauchet et al. 2015a). The hippocampal volume (sum of right and left sides), total cranial volume defined as the volume within the cranium including the brain, meninges, and CSF, and total white matter abnormalities (defined as T1 hypointensities and expressed in cm3) were used as outcomes. White matter abnormalities (WMA) were measured with FreeSurfer software. The segmentation process, subsequently extended to label white matter abnormalities, has been described in detail elsewhere (Fischl et al. 2002). WMA calculated on T1 images with this method has been shown to be highly correlated with manual and semi-manual measurements from T2/FLAIR (r > 0.93 when including extreme values; >0.72 when excluding extreme values) (Jacobs et al. 2013; Bagnato et al. 2003).
Statistics
The participants’ characteristics were summarized using means, standard deviations (SDs), frequencies and percentages, as appropriate. They were separated into two groups based on the increase or decrease of sway area of COP from open to closed eyes. Comparisons were performed using a chi-square, paired t test, or an independent samples t test as appropriate. A Pearson correlation was used to characterize the association between the relative difference between open and closed eyes of sway area and hippocampal volume. Multiple linear regression analysis was performed to examine the association between COP motion parameters (sway area, the path length of AP and ML displacement; dependent variables) and the hippocampal volume (independent variables) adjusted according to the participants’ characteristics (i.e., age, sex, body mass index, lower limb proprioception, distance vision, 15-item GDS score, total cranial volume, and white matter abnormalities). Overall alpha was set at <0.05.
Results
The mean age and standard deviation (SD) of the participants was 69.7 ± 3.4 years with 41.4 % women. Lower limb proprioception (/8) and distance vision (/10) scores were respectively 5.2 ± 1.2 and 8.3 ± 1.4 and the 15-items GDS score was 1.9 ± 2.2 (Table 1). There were no significant differences between the two groups (i.e., participants who increase or decrease their sway area of COP from open to closed eyes) for any of the clinical characteristics. Overall (i.e., for all participants), sway area decreased with closed eyes compared to open eyes but this decrease was not significant (172.8 ± 125.2 versus 190.2 ± 133.5 mm2 with P = 0.244). In contrast, path length of AP and ML displacement increased significantly with closed eyes compared to open eyes (291.9 ± 151.6 versus 261.6 ± 134.2 mm with P = 0.002, and 593.0 ± 341.8 versus 424.9 ± 185.5 mm with P < 0.001, respectively). Comparisons between groups showed that participants who decreased their sway area from open to closed eyes had a greater sway area with their eyes open (P = 0.032) and a lower sway area with their eyes closed (P = 0.002) than their counterparts. The relative difference for all participants in sway area from open to closed eyes was 9.4 ± 48.7 % (190.2 ± 133.5 mm2 with eyes open and 172.8 ± 125.2 mm2 with eyes closed). The sum of the right and left mean hippocampal volume was 7.7 ± 0.8 cm3. There was no significant difference in brain volume between the groups of participants.
Table 1.
Clinical, postural and brain volume characteristics of participants (n = 70)
| Total population (n = 70) | Relative differencea in sway area of COP between eyes open and closed conditions | P valueb | ||
|---|---|---|---|---|
| Decrease (n = 36) | Increase (n = 34) | |||
| Clinical characteristics | ||||
| Age (years), mean ± SD | 69.7 ± 3.4 | 69.4 ± 3.2 | 69.9 ± 3.7 | 0.572 |
| Female, n (%) | 29 (41.4) | 16 (44.4) | 13 (38.2) | 0.598 |
| Body mass index (kg/m2), mean ± SD | 25.6 ± 3.2 | 25.0 ± 3.0 | 26.1 ± 3.3 | 0.134 |
| Lower limb proprioceptionc score (/8), mean ± SD | 5.2 ± 1.1 | 5.4 ± 1.0 | 5.0 ± 1.1 | 0.202 |
| Distance visiond score (/10), mean ± SD | 8.4 ± 1.4 | 8.1 ± 1.5 | 8.6 ± 1.4 | 0.133 |
| 15-item Geriatric depression scale (15), mean ± SD | 1.9 ± 2.2 | 2.1 ± 1.9 | 1.7 ± 2.5 | 0.518 |
| COP parameters | ||||
| Sway areae, mean ± SD | ||||
| Open eyes (mm2) | 190.2 ± 133.5 | 223.4 ± 158.2 | 155.2 ± 90.8 | 0.032 |
| Closed eyes (mm2) | 172.8 ± 125.2 | 128.8 ± 84.7 | 219.4 ± 144.2 | 0.002 |
| Relative differencea (%) | 9.4 ± 48.7 | 47.4 ± 34.9 | −30.8 ± 20.5 | <0.001 |
| Path length of AP displacement, mean ± SD | ||||
| Open eyes (mm) | 261.6 ± 134.2 | 237.2 ± 86.2 | 294.9 ± 177.4 | 0.127 |
| Closed eyes (mm) | 291.9 ± 151.6 | 277.7 ± 127.4 | 311.2 ± 181.0 | 0.436 |
| Relative differencea (%) | −9.8 ± 19.6 | −12.5 ± 18.5 | −6.2 ± 20.8 | 0.253 |
| Path length ML displacement, mean ± SD | ||||
| Open eyes (mm) | 424.9 ± 185.5 | 400.2 ± 132.3 | 458.6 ± 239.4 | 0.266 |
| Closed eyes (mm) | 593.0 ± 341.8 | 546.1 ± 211.4 | 657.0 ± 463.2 | 0.252 |
| Relative differencea (%) | −28.4 ± 21.7 | −28.0 ± 22.0 | −28.9 ± 21.8 | 0.884 |
| Brain structure volumes (cm3) | ||||
| Total cranial volume | 1535.8 ± 134.6 | 1529.5 ± 142.1 | 1542.5 ± 127.9 | 0.687 |
| Total white matter abnormalitiesf | 2.6 ± 2.1 | 2.3 ± 1.3 | 3.0. ± 2.7 | 0.210 |
| Hippocampusg | 7.7 ± 0.8 | 7.6 ± 0.8 | 7.8 ± 0.8 | 0.179 |
P value (i.e., < 0.05) indicated in italic
CI confidence interval, COP center of pressure, AP anterior-posterior, ML medial-lateral
aCalculated from the formula (eyes open – eyes closed / ((eyes open + eyes closed) / 2) × 100
bComparison based on independent samples t-test or chi square, as appropriate
cSample mean value of left and right side and based on graduated diapason placed on the lower limb
dBinocular visual acuity at a distance of 5 m with a Snellen letter test chart
eSum of anterior-posterior and medial-lateral displacements
fDefined as T1 hypointensities and measured using FreeSurfer
gSum of left and right hippocampus
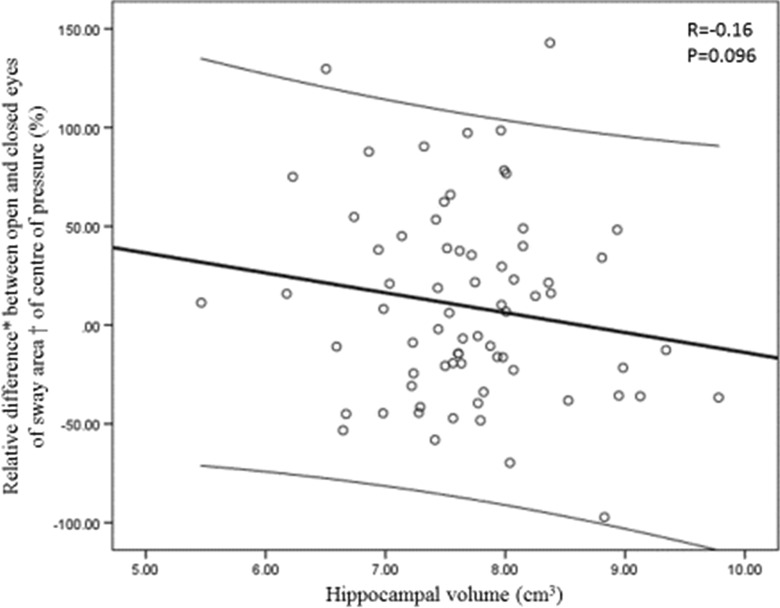
As illustrated in Fig. 1, there was a trend for a negative correlation between the relative difference in sway area and hippocampal volume (r = −0.16, P = 0.096), which underscores that increase in postural sway area when closed eyes (i.e., negative relative difference with high value) was associated with greater hippocampal volume. Table 2 displays the results of multiple linear regression analysis showing the association of COP motion parameters (sway area, AP and ML displacement; dependent variables) with hippocampal volume (independent variable) adjusted according to the participants’ characteristics. There was no significant association between the hippocampal volume and the separate eyes open and closed conditions for sway area (P = 0.117 and P = 0.252) and path length of ML displacement (P = 0.117 and P = 0.142). A trend for an increase in path length of AP displacement with greater hippocampal volume was shown (P = 0.075 for eyes open and P = 0.071 for eyes closed). In final, hippocampal volume was negatively associated with the difference in sway area from eyes open to eyes closed (β −18.21; P = 0.044). That means that an increase in sway area with eyes closed in comparison to eyes open was associated with greater hippocampal volume.
Fig. 1.
Relationship between the relative difference between open and closed eyes of sway area of center of pressure and hippocampal volume. The thick line is the best-fit linear regression line, and the thin lines at the top and bottom are the limits of the 95 % confidence interval. *calculated from the formula (eyes open – eyes closed / (eyes open + eyes closed) / 2) × 100. †sum of the total area of anterior-posterior and medial-lateral displacements
Table 2.
Multiple linear regression models showing the association between centre of pressure motion parameters (sway area, path length of anterior-posterior and medial-lateral displacement; dependent variables) and hippocampal volume (independent variable) adjusted on characteristics of participants (n = 70)
| COP motion parameters used as dependent variable in multiple linear regression | Open eyesa | Closed eyesa | Relative difference between open and closedb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ßc | 95 % CI | P value | ßc | 95 % CI | P value | ßc | 95 % CI | P value | |
| Sway aread | −39.40 | [−88.93; 10.14] | 0.117 | 26.86 | [−19.63; 73.35] | 0.252 | −18.21 | [−35.93; −0.48] | 0.044 |
| Path length of AP displacement | 47.03 | [−5.00; 99.06] | 0.075 | 53.17 | [−4.78; 111.12] | 0.071 | −2.86 | [−10.48; 4.77] | 0.453 |
| Path length of ML displacement | 58.43 | [−15.21; 132.07] | 0.117 | 99.02 | [−34.61; 232.64] | 0.142 | 1.38 | [−7.27; 10.03] | 0.750 |
Coefficient of regression (ß) and significant P value (i.e., P < 0.05) indicated in bold
AP anterior-posterior, COP centre of pressure, ML medial-lateral β coefficient of regression beta corresponding to an increase or a decrease in value of center of mass motion parameters, CI confidence interval
aSeparated models for open and closed eyes and ratio open eyes/closed eyes
bCalculated from the formula (eyes open – eyes closed / ((eyes open + eyes closed) / 2) × 100
cAdjusted on participant characteristics (age, sex, body mass index, lower limb proprioception score, distance vision score, and 15-item Geriatric Depression Scale) and brain volume characteristics (total cranial volume and total white matter abnormalities)
dSum of anterior-posterior and medial-lateral displacements
Discussion
Our findings show an association between hippocampal volume and postural sway in normal aging. A significant increase in the sway area of COP from open to closed eyes was associated with greater hippocampal volume, and a trend for an association between increased path length of AP displacement and greater hippocampal volume was also reported. These findings suggest that the hippocampus is involved in upright postural control in normal aging.
The role of the central nervous system in postural control has previously been found to be more related to subcortical than to cortical regions (Moro et al. 2010; Rinne et al. 2008; Hülsdünker et al. 2015). However, recent findings underscore the cortical contribution to postural control with a particular involvement of the frontal and the parietal cortices (Hülsdünker et al. 2015; Mihara et al. 2008; Van Impe et al. 2012; Kido et al. 2010). Our study demonstrates for the first time that the hippocampus is associated with maintaining upright stance in healthy older adults. Increase in upright postural sway was associated with greater hippocampal volume, showing indirectly that decrease in upright postural sway was associated with lower hippocampal volume. More precisely, compared to a referent condition, which was maintaining an upright position with open eyes, the challenging condition of closed eyes led to a decrease in postural sway area, this decrease being associated with lower hippocampal volume. Because lower hippocampal volume is considered to be an abnormal condition, this decrease in postural sway suggests an abnormal postural control, and therefore may be interpreted as being associated with increased instability (Bruijn et al. 2013; Borel and Alescio-Lautier 2014; Lord et al. 1991; Desai et al. 2010). Interestingly, postural instability is usually characterized by an increase in postural sway rather than a decrease (Borel and Alescio-Lautier 2014; Lord et al. 1991). However, from a biomechanical perspective, a certain amount of variability is necessary to maintain an upright posture (Bruijn et al. 2013; Beauchet et al. 2011). Indeed, the excursion of the COP moves randomly within a perimeter of stability during a stable upright position and is a reflection of the ability to maintain the body’s COG above its base of support (Bruijn et al. 2013; Borel and Alescio-Lautier 2014; Lord et al. 1991; Desai et al. 2010). Consequently, it is reasonable to suggest that the decrease in sway area of COP from the open to closed-eyes condition could be an early marker of upright postural dysfunction interpreted as an inappropriate overcompensation of static postural control (i.e., increased rigidity) related to lower hippocampal volume.
Our findings also underscore that differences between COP parameters occurred from open to closed eyes standing position. While sway area of COP decreased, path length of AP and ML displacement increased. This increase was in a normal range when compared to previous studies and has been previously considered as a normal motor behavior (Borel and Alescio-Lautier 2014). Indeed, the closed eyes condition is a stress condition corresponding to a deprivation of the visual input that destabilizes upright postural control and leads to an increase in postural sway (Borel Borel and Alescio-Lautier 2014; Lord et al. 1991). The opposite behavior reported with the sway area suggests that the control of this parameter is different and/or provides complementary information compared to the others. Indeed, the sway area compared to path length of AP and ML displacement is a global parameter encompassing the others, which could make this parameter more sensitive to change in postural control. In addition, it is important to underline that hippocampal volume of the studied population is in a normal range. It has been reported that the mean hippocampal volume obtained from the western population varies from 5.6 to 7.8 cm3 (Honeycutt and Smith 1995; Pruessner et al. 2000; Szabo et al. 2001). This information confirms that the change in upright postural control reported in our study may reflect the onset of an abnormal postural control.
The association between standing postural sway and hippocampal volume is contradictory to those of previous studies that have shown the role of the hippocampus in gait stability, gait being considered as a particular condition of dynamic balance (Beauchet et al. 2011; Beauchet et al. 2015a, b). With respect to the dynamic postural control of gait, stride width values (which are associated with dynamic balance) have not been associated with hippocampal volume (Beauchet et al. 2015a, b). The discrepancy with the results of the present study likely suggests that the hippocampus may have a different role in static and dynamic postural control.
It is important to note that our study has several limitations. First, the number of participants was small. Second, while this study is the first to demonstrate an association between hippocampal volume and static postural control, the cross-sectional design does not afford a causal relationship. Third, all participants were referred for the evaluation of a memory complaint, which prevents generalization to the entire older adult population. Further studies should include a combined assessment of postural control and memory performance to understand the relationship between hippocampal volume, postural control, and memory. Fourth, the foot frame used when assessing the body sway in our study may explain in part the decrease in body sway because it could be suggested that more attention was given to this extra information when the eyes were closed.
Conclusion
We found an association between an increase in upright postural sway area of COP from open to closed eyes and greater hippocampal volume in healthy older adults. This result suggests the involvement of the hippocampus in the upright postural control of aging and that clinicians should assess upright postural control in older adults with hippocampal abnormalities.
Acknowledgments
We acknowledge all participants included in the present study.
Author contributions
Conceived and designed the experiments: OB and GA. Performed the experiments: OB. Analyzed the data: OB and GA. Contributed reagents/materials/analysis tools: OB. Wrote the paper: OB, JB, TLA, VLC, TS and GA.
Compliance with ethical standards
Conflict of interest
The authors declare no relevant conflict of interest.
Funding
The study was financially supported by the French Ministry of Health (Projet Hospitalier de Recherche Clinique national n2009-A00533-54).
References
- Bagnato F, Jeffries N, Richert ND, Stone RD, Ohayon JM, McFarland HF, Frank JA. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782–1789. doi: 10.1093/brain/awg182. [DOI] [PubMed] [Google Scholar]
- Bast T, Feldon J. Hippocampal modulation of sensorimotor processes. Prog Neurobiol. 2003;70:319–345. doi: 10.1016/S0301-0082(03)00112-6. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Annweiler C, Verghese J, Fantino B, Herrmann FR, Allali G. Biology of gait control: vitamin D involvement. Neurology. 2011;76:1617–1622. doi: 10.1212/WNL.0b013e318219fb08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O, Launay CP, Annweiler C, Allali G. Hippocampal volume, early cognitive decline and gait variability: which association? Exp Gerontol. 2015;61:98–104. doi: 10.1016/j.exger.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Levinoff E, Allali G (2015b). Decrease in upright postural sway from open to closed eyes: episodic memory impairment matters too. J Am Geriatr Soc (in press) [DOI] [PubMed]
- Borel L, Alescio-Lautier B. Posture and cognition in the elderly: interaction and contribution to the rehabilitation strategies. Neurophysiol Clin. 2014;44:95–107. doi: 10.1016/j.neucli.2013.10.129. [DOI] [PubMed] [Google Scholar]
- Brown EC, Casey A, Fisch RI, Neuringer C. Trail making test as a screening device for the detection of brain damage. J Consult Psychol. 1958;22:469–474. doi: 10.1037/h0039980. [DOI] [PubMed] [Google Scholar]
- Bruijn SM, Meijer OG, Beek PJ, van Dieën JH. Assessing the stability of human locomotion: a review of current measures. J R Soc Interface. 2013;10:20120999. doi: 10.1098/rsif.2012.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A, Goodman V, Kapadia N, Shay BL, Szturm T. Relationship between dynamic balance measures and functional performance in community-dwelling elderly people. Phys Ther. 2010;90:748–760. doi: 10.2522/ptj.20090100. [DOI] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/WNL.55.11.1621. [DOI] [PubMed] [Google Scholar]
- Dubois B, Sarazin M, Lehericy S (2009) P2a-4 etude hippocampe: evaluation de l’efficacité du donépézil versus placebo sur des marqueurs IRM et cliniques chez des patients présentant des troubles cognitifs légers. Rev Neurol (Paris) 165:66–67
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Wholebrain segmentation: automated labeling of neuroanatomical structures in the humanbrain. Neuron. 2002;33:341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental state“. A Practical Method for Grading the Cognitive State of Patients for the Clinician J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Honeycutt NA, Smith CD. Hippocampal volume measurements using magnetic resonance imaging in normal young adults. J Neuroimaging. 1995;5:95–100. doi: 10.1111/jon19955295. [DOI] [PubMed] [Google Scholar]
- Hülsdünker T, Mierau A, Neeb C, Kleinöder H, Strüder HK. Cortical processes associated with continuous balance control as revealed by EEG spectral power. Neurosci Lett. 2015;592:1–5. doi: 10.1016/j.neulet.2015.02.049. [DOI] [PubMed] [Google Scholar]
- Jacobs HI, Leritz EC, Williams VJ, Van Boxtel MP, van der Elst W, Jolles J, Verhey FR, McGlinchey RE, Milberg WP, Salat DH. Association between white matter microstructure, executive functions, and processing speed in older adults: the impact of vascular health. Hum Brain Mapp. 2013;34:77–95. doi: 10.1002/hbm.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido T, Tabara Y, Igase M, Ochi N, Uetani E, Nagai T. Postural instability is associated with brain atrophy and cognitive impairment in the elderly: the J-SHIPP study. Dement Geriatr Cogn Disord. 2010;29:379–387. doi: 10.1159/000255106. [DOI] [PubMed] [Google Scholar]
- Launay C, De Decker L, Annweiler C, Kabeshova A, Fantino B, Beauchet O. Association of depressive symptoms with recurrent falls: a cross-sectional elderly population based study and a systematic review. J Nutr Health Aging. 2013;17:152–157. doi: 10.1007/s12603-012-0370-z. [DOI] [PubMed] [Google Scholar]
- Leandri M, Cammisuli S, Cammarata S, Baratto L, Campbell J, Simonini M, Tabaton M. Balance features in Alzheimer’s disease and amnestic mild cognitive impairment. J Alzheimers Dis. 2009;16:113–120. doi: 10.3233/JAD-2009-0928. [DOI] [PubMed] [Google Scholar]
- Lord SR, Clark RD, Webster IW. Postural stability and associated physiological factors in a population of aged persons. J Gerontol. 1991;46:M69–M76. doi: 10.1093/geronj/46.3.M69. [DOI] [PubMed] [Google Scholar]
- Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Role of the prefrontal cortex in human balance control. NeuroImage. 2008;43:329–336. doi: 10.1016/j.neuroimage.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, Lozano AM. Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain. 2010;133:215e224. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- Pérès K, Chrysostome V, Fabrigoule C, Orgogozo JM, Dartigues JF, Barberger-Gateau P. Restriction in complex activities of daily living in MCI: impact on outcome. Neurology. 2006;67:461–466. doi: 10.1212/01.wnl.0000228228.70065.f1. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Ma SY, Lee MS, Collan Y, Roytta M. Loss of cholinergic neurons in the pedunculopontine nucleus in Parkinson’s disease is related to disability of the patients. Parkinsonism Relat Disord. 2008;14:553e557. doi: 10.1016/j.parkreldis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Leutgeb S, Leutgeb JK. Spatial and memory circuits in the medial entorhinal cortex. Curr Opin Neurobiol. 2014;32C:16–23. doi: 10.1016/j.conb.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- Szabo CA, Xiong J, Lancaster JL, Rainey L, Fox P. Amygdalar and hippocampal volumetry in control participants: differences regarding handedness. AJNR Am J Neuroradiol. 2001;22:1342–1345. [PMC free article] [PubMed] [Google Scholar]
- Tangen GG, Engedal K, Bergland A, Moger TA, Mengshoel AM. Relationships between balance and cognition in patients with subjective cognitive impairment, mild cognitive impairment, and Alzheimer disease. Phys Ther. 2014;1994:1123–1134. doi: 10.2522/ptj.20130298. [DOI] [PubMed] [Google Scholar]
- Van der Linden M, Coyette F, Poitrenaud F, Kalafat M, Calicis F, Adam F (2004). L’épreuve de rappel libre/rappel indicé à 16 items (RL/RI-16). In L’évaluation des troubles de la mémoire, (ed. Solal), Marseille
- Van Impe A, Coxon JP, Goble DJ, Doumas M, Swinnen SP. White matter fractional anisotropy predicts balance performance in older adults. Neurobiol Aging. 2012;33:1900–1912. doi: 10.1016/j.neurobiolaging.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WMS-R: Wechsler Memory Scale - revised manual. San Antonio, TX: The Psychological Corporation, Harcourt Brace Jovanovich; 1987. [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on mild cognitive impairment. J Inter Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]