Abstract
In dramatic contrast to rats on a control diet, rats maintained on a high-fat diet (HFD) failed to activate brown adipose tissue (BAT) during cooling despite robust increases in their BAT activity following direct activation of their BAT sympathetic premotor neurons in the raphe pallidus. Cervical vagotomy or blockade of glutamate receptors in the nucleus of the tractus solitarii (NTS) reversed the HFD-induced inhibition of cold-evoked BAT activity. Thus, a HFD does not prevent rats from mounting a robust, centrally driven BAT thermogenesis; however, a HFD does alter a vagal afferent input to NTS neurons, thereby preventing the normal activation of BAT thermogenesis to cooling. These results, paralleling the absence of cooling-evoked glucose uptake in the BAT of obese humans, reveal a neural mechanism through which consumption of a HFD contributes to reduced energy expenditure and thus to weight gain.
Keywords: obesity, vagus nerve, nucleus of the solitary tract
exposure to a cool environment increases the sympathetic outflow to brown adipose tissue (BAT), providing the essential thermoregulatory drive to stimulate metabolism and heat production in mammalian BAT, including that in adult humans (5, 18, 24, 31, 33). A wide array of nonthermoregulatory factors (e.g., prostaglandins, leptin, triiodothyronine, and lipids) can also act in the CNS, directly in BAT, or through vagal afferents to influence BAT thermogenesis (reviewed in Ref. 16). Through its consumption of lipid energy stores, thermogenic metabolism in BAT is a neurally regulated contributor to energy homeostasis. Thus, particularly in the face of an elevated consumption of energy-rich foods, a chronically reduced level of BAT activity would contribute to the augmented adipose energy stores that characterize obesity.
Indeed, mice without BAT exhibit a propensity for obesity and diabetes (8, 13), and conversely, overexpression of uncoupling protein-1 (UCP-1), which is principally responsible for thermogenesis in BAT, protects against high fat diet (HFD)-induced obesity (9). The consistent findings that obese humans have a lower incidence of metabolically active BAT (5, 20, 24, 31) and that the basal sympathetic activation of BAT is reduced in rats chronically maintained on a HFD (10, 25) also supports a role for reduced BAT activity in the excess adipose accumulation of obesity. Seemingly at odds with these observations, UCP-1 is upregulated in HFD-induced obese rodents (reviewed in Ref. 7), suggesting a more active BAT in this model.
To determine directly the effect of HFD on the sympathetic activation of BAT, we tested the basic thermoregulatory cold-defensive activation of BAT in rats maintained on a HFD. Mimicking the low, cooling-evoked BAT uptake of [18F]fluorodeoxyglucose in obese humans, high-fat-fed rats had almost no increase in BAT activity in response to skin and core cooling. The abrupt return of cooling-evoked BAT activity following interruption of vagal afferent activity points to an altered vagal BAT inhibitory input (17) as the primary neurobiological mechanism underlying the impairment of BAT activation in rats on a HFD.
MATERIALS AND METHODS
All procedures conformed to the regulations detailed in the Guide for the Care and Use of Laboratory Animals: Eighth Edition (National Research Council, National Academies Press, 2010) and were approved by the Animal Care and Use Committee of the Oregon Health and Science University.
Male and female Sprague-Dawley rats (Charles River Laboratories, Indianapolis, IN) were housed in a colony room maintained at 22–23°C with a 12:12-h light-dark cycle. Rats were fed a control diet (13% kcal from fat; Laboratory Rodent Diet 5001, LabDiet.com) until they weighed between 275 and 350 g, at which time they were transferred to one of two control diets (10% kcal from fat; Laboratory Rodent Diet 5001 or Research Diets D12450H) or a HFD (45% kcal from fat; Research Diets D1245). Rats were maintained on these diets for ≥60 days prior to experimentation. No differences were noted between responses in rats maintained on the two control diets, and therefore, these data were considered as a single group for statistical comparisons. For the rats maintained on a HFD, we observed the recognized variability in weight gain, usually described as resistant, middle, and obese groups based on tertiles of weight gain (27). Since there were no differences in the BAT thermogenic responses when the data were considered as three groups, all rats on the HFD were considered as a single group for statistical comparisons.
For acute physiology experiments, rats were anesthetized with isoflurane (2–3% in 100% O2) for cannulation of the trachea and femoral artery and vein before being transitioned to urethane (750 mg/kg iv) and α-chloralose (60 mg/kg iv) anesthesia. Rats were artificially ventilated (100% O2) and paralyzed with d-tubocurarine. Rats were placed in a stereotaxic frame and thermocouples inserted into the rectum for core body temperature (TCORE), into the left interscapular BAT pad for BAT temperature (TBAT), and onto the hindquarter skin under the thermal blanket for skin temperature (TSKIN). A sympathetic nerve innervating the right interscapular BAT pad was recorded using bipolar hook electrodes (15). BAT sympathetic nerve activity (SNA) was amplified (x10K, 1–300 Hz, CyberAmp 380; Axon Instruments) and digitized to a hard drive (Spike 2; Cambridge Electronic Design) along with all other variables.
Initially, both TCORE and TSKIN were maintained >36.0°C with a heat lamp and perfusion of the thermal blanket with warm water. Rats were cooled by turning off the heat lamp and perfusing the thermal blanket with cool water to reach a target skin temperature of 36.0 to 35.0°C. During the skin cooling, BAT SNA, TBAT, TCORE, and expired CO2 were considered as dependent variables and were allowed to change freely. Using Spike 2 software (CED), a continuous measurement (4-s bins) of BAT SNA amplitude was obtained as the root mean square value of the BAT SNA (square root of the total power in the 0.1 to 20 Hz band) from the autospectra of sequential 4-s segments of BAT SNA. The baseline level of BAT SNA was taken as the mean BAT SNA amplitude during a 2-min period of minimum BAT SNA recorded when the rat was in a warm condition (TCORE and TSKIN >36.5°C) and basal BAT SNA was absent. For the cooling response, statistical comparisons were made between groups, using the values of the individual variables over the 30-s period when the TSKIN reached a nadir of 35.5 ± 0.5°C. In several rats maintained on a control diet, a TSKIN of 35.5°C was not attained, since the robust activation of cooling-evoked BAT thermogenesis counteracted the fall in TSKIN. After the target TSKIN was attained, a subset of the rats maintained on a HFD (n = 5) received bilateral nanoinjections of a glutamate receptor antagonist (kynurenate, 80 nl, 0.1 M, or a combination of AP5/CNQX 80nl, 5:5 mM) into the NTS (coordinates from calamus scriptorius: 1.2 mm rostral, 1.0 mm lateral, and 0.6 mm ventral). Another subset of rats maintained on a HFD (n = 5) received either bilateral nanoinjections of vehicle (saline, 60 nl; n = 2) or no nanoinjection (n = 3). The responses in the vehicle-injected control rats and the rats receiving no injection were similar, so these rats were considered a single group for statistical comparisons. Statistical comparisons were made between the 30-s period just prior to the NTS nanoinjections and the peak 30-s values of the individual variables within 30 min of the nanoinjection or for controls receiving no injection within 30 min of reaching the target TSKIN of 35.5°C. In another group (n = 5) of HFD rats, bilateral cervical vagotomies were performed at the time of tracheal cannulation prior to undergoing the protocol and cooling procedures identical to all other vagus-intact rats. To section the cervical vagi, a midline incision was made on the ventral surface of the neck, and the muscles overlying the common carotid arteries were separated with blunt dissection to expose the cervical vagi. Subsequently, an ∼4-mm portion of each cervical vagus was dissected from each common carotid artery in the region just rostral to the clavicle. A suture was placed around each vagus nerve, and after slight retraction, the vagi were sectioned completely using angled iridectomy scissors, and ∼2 mm of vagus nerve was then sectioned from the proximal end of the sectioned vagus. In every case, this simple procedure produced a complete section of the cervical vagi that was easily verified visually as well as by the marked slowing in respiratory rate and increase in heart rate following the section of the second vagus. For the responses evoked by nanoinjection of the GABAA antagonis bicuculline (Bic) in the rostral raphe pallidus area (rRPa; coordinates from calamus scriptorius: 2.8–3.0 mm rostral on the midline, 3.0 mm ventral), statistical comparisons were made between groups using the difference between the 30-s period just prior to the injection and the peak 30-s values of the individual variables within 15 min of the injection.
All statistics were performed using Systat Software (version 10; Cranes Software International). Data are expressed as means ± SE. Statistical significance was assessed using a paired t-Test or ANOVA. Post hoc comparisons were performed using layered Bonferroni corrections. Results with P < 0.05 were considered significant.
RESULTS
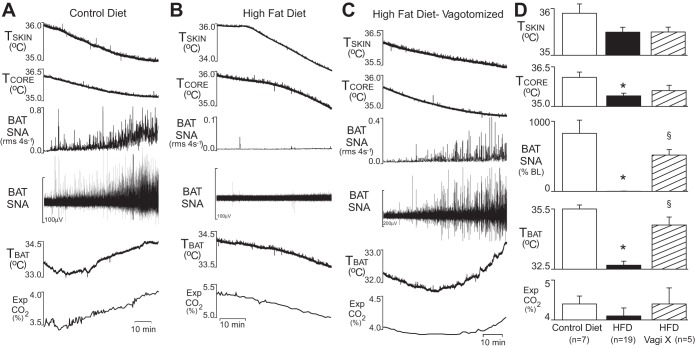
To test the hypothesis that cooling-evoked increases in BAT SNA and BAT thermogenesis are impaired in rats maintained on a HFD compared with rats maintained on a control diet, BAT SNA and TBAT were measured during skin cooling. Since activation of vagal afferent nerve fibers can inhibit BAT SNA (17) and vagal afferent activity is responsive to dietary composition (22, 28), we also determined whether vagal afferent activity is required for a HFD-induced impairment of cooling-evoked BAT SNA and BAT thermogenesis. In rats maintained on the control diet, skin cooling increased BAT SNA dramatically (+828 ± 188% of baseline, n = 7, P < 0.01; Fig. 1A). In sharp contrast, rats maintained on a HFD and with intact vagus nerves failed to increase their BAT SNA (+2 ± 2% of baseline, n = 19; Fig. 1B) to skin cooling. However, in rats that were maintained on a HFD but acutely vagotomized immediately prior to BAT SNA recordings, skin cooling elicited a robust increase in BAT SNA (+519 ± 78% of baseline, n = 5, P < 0.01; Fig. 1C). The TSKIN attained by circulating cool water through a cooling blanket surrounding the rat's trunk was not significantly different among groups (control diet: 35.9 ± 0.2°C; HFD: 35.5 ± 0.1°C; HFD with vagotomy: 35.5 ± 0.1°C). As expected, TBAT initially decreased with skin cooling in all groups; however, the sympathetic activation of BAT thermogenesis that occurred in the control diet and in the vagotomized HFD groups resulted in levels of TBAT that were significantly higher than that in the vagus intact HFD group (35.5 ± 0.2 and 34.7 ± 0.4°C vs. 32.7 ± 0.2°C, respectively, P < 0.001).
Fig. 1.
Skin cooling increased brown adipose tissue (BAT) thermogenesis in rats on a control diet and in acutely vagotomized rats on a high-fat diet (HFD) but not in rats with intact vagi on a HFD. A–C: temperatures of the skin (TSKIN), core (TCORE), and BAT (TBAT), the integrated (root mean square/4s) and raw BAT sympathetic nerve activity (SNA), and the expired CO2 (Exp CO2) during a skin-cooling episode in a rat maintained on a control diet (A), on a HFD (B), and on a HFD but with an acute bilateral cervical vagotomy (C). D: group data representing the mean ± SE of the peak values during episodes of skin cooling. *P < 0.05 compared with the control diet group; §P < 0.05 compared with HFD. %BL, %baseline; Vagi X, bilateral cervical vagotomy.
During skin cooling, TCORE decreased in all groups but was sustained at a higher level in the control diet group compared with the HFD group (36.1 ± 0.2 vs. 35.4 ± 0.1°C, respectively, P < 0.01), which was likely due to differences in BAT thermogenesis. The TCORE of the vagotomized HFD rats (35.6 ± 0.2°C) did not differ from that in either the control diet or the HFD rats (P = 0.078 and P = 0.433, respectively). Expired CO2 during cooling did not differ between the control diet and HFD groups (control diet: 4.4 ± 0.2% vs. HFD: 4.1 ± 0.2%, HFD vagotomized: 4.4 ± 0.4%).
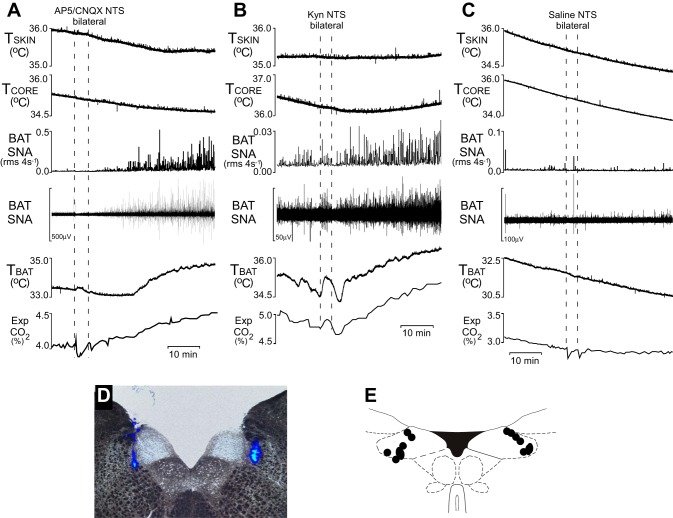
In rats maintained on a HFD and with intact vagi, bilateral nanoinjections (60 nl) of the glutamate receptor antagonists (2R)-amino-5-phosphonovaleric acid (AP5; 5 mM) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 5 mM) or kynurenate into the NTS (Fig. 2C) restored the activation of BAT SNA and TBAT to skin cooling (Fig. 2, A and B). As shown at the beginning of the traces in Fig. 2, A–C, and in Fig. 1B, skin cooling failed to increase BAT SNA or TBAT in intact HFD rats. Furthermore, as shown in Fig. 2C, injection of saline vehicle into the NTS did not restore the activation of BAT SNA (94 ± 4% of baseline prior to injection of saline and 94 ± 7% of baseline after injection of saline, P = 0.986, n = 5). However, within a few minutes after blockade of glutamate receptors in NTS, BAT SNA increased significantly from 106 ± 2 to 502 ± 82% of baseline (P < 0.01, n = 5), i.e., to levels comparable with the cooling-evoked increases in BAT SNA in rats on a control diet or in vagotomized HFD rats. The apparent relief of a vagally mediated inhibition of cooling-evoked BAT SNA following blockade of glutamate receptors in NTS also resulted in a return of cooling-evoked BAT thermogenesis; TBAT rose from 32.9 ± 0.5 to 34.8 ± 0.4°C (P < 0.01, n = 5) after nanoinjections of ionotropic glutamate receptor antagonists into the NTS (Fig. 2, A and B). In contrast, TBAT continued to fall from 32.5 ± 0.2°C prior to the nanoinjections of saline into the NTS to 31.5 ± 0.3°C (P < 0.01, n = 5) 30 min after these nanoinjections (Fig. 2C).
Fig. 2.
Blockade of glutamate receptors in the nucleus of the tractus solitarii (NTS) restores skin cooling-evoked activation of BAT in rats maintained on a HFD. Representative examples of the skin cooling-evoked increases in BAT SNA and TBAT following bilateral nanoinjections of the glutamate receptor antagonists (2R)-amino-5-phosphonovaleric acid (AP5) and and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (A) or kynurenate (B) in the NTS in 2 rats maintained on a HFD. C: representative example of the absence of skin cooling-evoked increases in BAT SNA and TBAT following bilateral nanoinjections of saline in the NTS in a rat maintained on a HFD. D: histological coronal section illustrating typical bilateral injection sites in the NTS. E: a composite mapping of the centers of the NTS injection sites in 7 rats plotted on an atlas drawing through the rat caudal medulla (21).
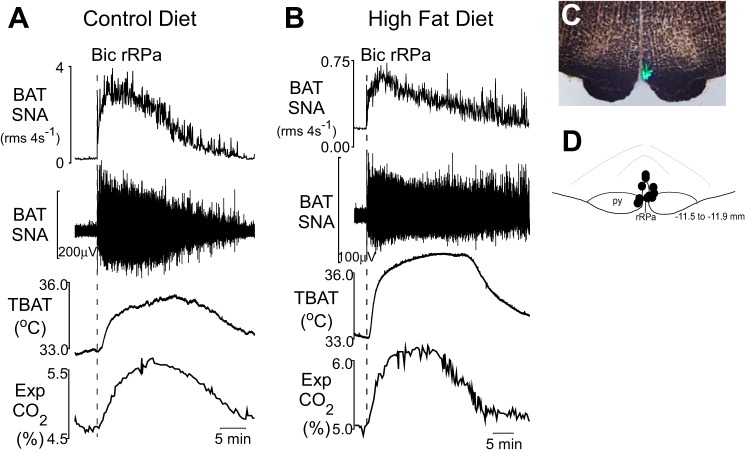
To assess a potential difference in the functionality of the neural pathway between the BAT sympathetic premotor neurons (17) in the rRPa and the interscapular BAT in rats maintained on a HFD, the GABAA receptor antagonist Bic was nanoinjected in the rRPa (Fig. 3, C and D) of rats maintained on control diet (n = 7) and rats maintained on HFD (n = 5). Bic in the rRPa increased BAT SNA in both groups, with the increase being larger in rats on the control diet compared with those on the HFD (+1,409 ± 144% of baseline in rats on control diet compared with +481 ± 105% of baseline in rats on HFD, P < 0.05; Fig. 3, A and B). The increases in TBAT evoked by Bic in the rRPa were not different between rats on the control diet (3.0 ± 0.5°C) and those on HFD (3.5 ± 0.4°C). The Bic-evoked increase in expired CO2 was significantly different between groups (+1.0 ± 0.1% in control diet rats vs. +1.3 ± 0.1% in HFD rats, P < 0.05).
Fig. 3.
Blockade of GABAA receptors in the rostral raphe pallidus area (rRPa) increases BAT activity in all rats whether maintained on a control diet or on a HFD. A and B: nanoinjection of the GABAA receptor antagonist bicuculline (Bic) in the rRPa increases BAT SNA, TBAT, and expired CO2 in a rat maintained on a control diet (A) and in a rat maintained on a HFD (B). C: histological coronal section illustrating an injection site in the rRPa. D: a composite mapping of the centers of the rRPa injection sites in 12 rats plotted on an atlas drawing through the rat rostral medulla (21).
DISCUSSION
Since the ambient temperature in which rats are maintained is normally below their thermoneutral zone, their cooling-evoked activation of BAT makes a tonic contribution to their overall energy expenditure. The major finding of this study is that maintenance on a HFD markedly reduces the cooling-evoked increases in BAT SNA and BAT thermogenesis observed in rats on a control diet. This reduction in BAT activity is dependent on the glutamatergic excitation of NTS neurons by a population of vagal afferents. Thus, consumption of a HFD enhances a vagal afferent mechanism that chronically reduces BAT energy expenditure and thereby contributes to HFD obesity. The consistent finding that the cooling-evoked activation of BAT is markedly diminished in obese humans (5, 20, 24, 31) raises the striking possibility that this parallel reduction in human BAT energy expenditure also arises from an enhanced vagal afferent mechanism due to exposure to a HFD.
Vagal nerve section or blockade of glutamate receptors in the NTS reversed the HFD-induced inhibition of cooling-evoked increases in BAT SNA and BAT thermogenesis, implicating an active glutamatergic excitation of a population of second-order vagal viscerosensory NTS neurons in the central neural circuit mechanism underlying the HFD-induced abrogation of cooling-evoked BAT thermogenesis. The NTS is the termination site of vagal afferents, and glutamate is their primary neurotransmitter (2), and activation of neurons in the NTS produces an inhibition of BAT SNA and BAT thermogenesis (4, 30). Diverse populations of vagal afferents can influence the regulation BAT; both activation and inhibition of BAT SNA can be evoked by electrical stimulation of vagal afferents (17). Short-term consumption of a HFD increases sympathetic activation of BAT (11) likely via vagal afferents that are activated by lipid in the duodenum (3). In contrast, long-term maintenance on a HFD, a model reflecting the state of many in the obese human population, results in impaired BAT activity (current study and Ref. 10), which is likely due to vagal activation of BAT sympathoinhibitory neurons in NTS (4, 30).
HFD could alter either the level of discharge of the relevant vagal afferent and/or the responsiveness of its NTS target neurons, including via a potential glial modulation of glutamatergic transmission in NTS (32) or via an augmented glutamate release from vagal afferent terminals due to lipid modulation of their TRPV1 conductance (1). The relevant population of vagal afferents required for the effects of HFD on BAT activation, as well as the nature of the stimulus to which they respond, which is potentially augmented in HFD rats, remains to be identified. Of interest are the hepatic afferents responsible for the decrease in BAT thermogenesis following the upregulation of hepatic glucokinase during high-fat feeding (29). Another possibility is that activation of peroxisome proliferator-activated receptor gamma (PPARγ), a nuclear receptor for dietary-derived lipids that is found in the nodose ganglion cell bodies of vagal afferents (12), drives the altered vagal afferent activity that results in an impaired BAT SNA and BAT thermogenesis. In this regard, chronic administration of PPAR receptor agonists decreases norepinephrine turnover in BAT, reduces oxygen consumption, and increases weight gain (6). Knockout of PPARγ in Phox2b-expressing vagal afferent neurons in the nodose ganglion led to elevated oxygen consumption and heat production in HFD mice (12), consistent with the potential for PPARγ activation in primary vagal sensory neurons to inhibit BAT thermogenesis. Although PPARγ mRNA levels in nodose ganglion were reduced in HFD mice (12), the elevated levels of dietary-derived lipids during exposure to a HFD may be sufficient to induce an inhibition of BAT thermogenesis, even in the face of PPARγ mRNA downregulation in nodose ganglion neurons.
The finding that blockade of GABAA receptors in rRPa significantly increased BAT SNA, independent of diet, indicates that a HFD does not eliminate all nonthermoregulatory (i.e., tonic) excitatory inputs to BAT sympathetic premotor neurons in rRPa (14), nor does it result in a sufficiently strong inhibition of BAT sympathetic preganglionic or ganglion neurons such that all excitations of BAT SNA via stimulation of BAT sympathetic premotor neurons are blocked in HFD-fed rats. However, the determination that the level of BAT SNA evoked by disinhibition of BAT sympathetic premotor neurons in rRPa was smaller in rats on a HFD compared with rats on a control diet does suggest that a HFD alters the relative levels of tonic excitation and inhibition at site(s) in the BAT sympathoexcitatory pathway from the BAT sympathetic premotor neurons in rRPa to the BAT ganglion cells. In this regard, our data would be consistent with a scenario in which a HFD increased a tonic or vagally mediated inhibition of BAT sympathetic preganglionic or ganglion neurons to a degree that was sufficient to block the cold-evoked increase in BAT SNA but only reduced that evoked by disinhibition of neurons in rRPa. In this regard, the neural circuit mechanism through which vagal stimulation (17) or NTS neurons (4, 30) can inhibit BAT SNA has not yet been determined. Alternatively, since the quantification of SNA is strongly influenced by the contact between the nerve and the recording electrodes, the difference in BAT SNA responses to Bic in rRPa could arise from differences in the “adipose insulation” on the BAT nerve bundles between the rats on HFD and on control diet, although there were no obvious differences noted during the surgical isolation of the nerves.
Although disinhibition of neurons in rRPa in rats on a HFD evoked a smaller increase in BAT SNA than in rats on a control diet, the evoked increase in BAT thermogenesis (monitored via TBAT) was not different between rats on a HFD and those on a control diet, and the Bic-evoked increase in expired CO2 (an index of metabolism) was larger in rats on HFD than in those on control diet. These data are consistent with an enhanced effect of BAT SNA on BAT thermogenesis and on BAT energy expenditure in rats on a HFD. They also suggest that stimuli capable of overcoming the HFD-induced abrogation of cooling-evoked BAT activation would result in potentiated BAT thermogenesis, consistent with the findings that norepinephrine-evoked increases in oxygen consumption are increased by a cafeteria diet (23) and that UCP-1 expression in BAT is increased by HFD (7).
Novel mechanisms to upregulate BAT energy expenditure for obesity therapy have focused on increasing the expression of UCP-1 in adipose tissue. Although this approach would increase the metabolic capacity of adipose tissue, a major implication of the current study is that upregulation of UCP-1 in adipose tissue, as seen in diet-induced obesity (7), might not significantly increase BAT energy expenditure, since the HFD also impairs activation of BAT sympathetic outflow. A similar critical point was made by the demonstration that PPARγ receptor agonists increased UCP-1 expression in adipose tissue but failed to increase energy expenditure (19, 26). The current studies demonstrate that vagal afferent activation of neurons in the NTS is essential for the HFD-induced impairment of BAT activation. These data provide an important foundation for defining potential targets for therapeutic approaches to reverse these HFD-induced impairments and thereby increase fat metabolism in obesity.
GRANTS
This work was supported by American Diabetes Association Basic Science Grant no. 1-13-BS-120 (C. J. Madden) and National Institutes of Health Grant R01-NS-091066 (S. F. Morrison).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.J.M. and S.F.M. conception and design of research; C.J.M. performed experiments; C.J.M. analyzed data; C.J.M. and S.F.M. interpreted results of experiments; C.J.M. and S.F.M. prepared figures; C.J.M. drafted manuscript; C.J.M. and S.F.M. edited and revised manuscript; C.J.M. and S.F.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Rubing Xing for excellent technical assistance.
REFERENCES
- 1.Andresen MC, Fawley JA, Hofmann ME. Peptide and lipid modulation of glutamatergic afferent synaptic transmission in the solitary tract nucleus. Front Neurosci 6: 191, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andresen MC, Kunze DL. Nucleus tractus solitarius—gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Blouet C, Schwartz GJ. Duodenal lipid sensing activates vagal afferents to regulate non-shivering brown fat thermogenesis in rats. PLoS One 7: e51898, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao WH, Madden CJ, Morrison SF. Inhibition of brown adipose tissue thermogenesis by neurons in the ventrolateral medulla and in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 299: R277–R290, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Festuccia WT, Oztezcan S, Laplante M, Berthiaume M, Michel C, Dohgu S, Denis RG, Brito MN, Brito NA, Miller DS, Banks WA, Bartness TJ, Richard D, Deshaies Y. Peroxisome proliferator-activated receptor-gamma-mediated positive energy balance in the rat is associated with reduced sympathetic drive to adipose tissues and thyroid status. Endocrinology 149: 2121–2130, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Fromme T, Klingenspor M. Uncoupling protein 1 expression and high-fat diets. Am J Physiol Regul Integr Comp Physiol 300: R1–R8, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Hamann A, Flier JS, Lowell BB. Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology 137: 21–29, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Kopecky J, Rossmeisl M, Hodny Z, Syrovy I, Horakova M, Kolarova P. Reduction of dietary obesity in aP2-Ucp transgenic mice: mechanism and adipose tissue morphology. Am J Physiol Endocrinol Metab 270: E776–E786, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Levin BE, Finnegan M, Triscari J, Sullivan AC. Brown adipose and metabolic features of chronic diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 248: R717–R723, 1985. [DOI] [PubMed] [Google Scholar]
- 11.Levin BE, Triscari J, Sullivan AC. Altered sympathetic activity during development of diet-induced obesity in rat. Am J Physiol Regul Integr Comp Physiol 244: R347–R355, 1983. [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Bookout AL, Lee S, Sun K, Jia L, Lee C, Udit S, Deng Y, Scherer PE, Mangelsdorf DJ, Gautron L, Elmquist JK. PPARgamma in vagal neurons regulates high-fat diet induced thermogenesis. Cell Metab 19: 722–730, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366: 740–742, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Madden CJ, Morrison SF. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neuroscience 122: 5–15, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 296: R831–R843, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison SF, Madden CJ. Central nervous system regulation of brown adipose tissue. Comp Physiol 4: 1677–1713, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab 19: 741–756, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293: E444–E452, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Nedergaard J, Cannon B. UCP1 mRNA does not produce heat. Biochim Biophys Acta 1831: 943–949, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Orava J, Nuutila P, Noponen T, Parkkola R, Viljanen T, Enerbäck S, Rissanen A, Pietiläinen KH, Virtanen KA. Blunted metabolic responses to cold and insulin stimulation in brown adipose tissue of obese humans. Obesity (Silver Spring) 21: 2279–2287, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney, Australia: Academic, 2007. [Google Scholar]
- 22.Raybould HE. Nutrient tasting and signaling mechanisms in the gut. I. Sensing of lipid by the intestinal mucosa. Am J Physiol Gastrointest Liver Physiol 277: G751–G755, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature 281: 31–35, 1979. [DOI] [PubMed] [Google Scholar]
- 24.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58: 1526–1531, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakaguchi T, Arase K, Fisler JS, Bray GA. Effect of a high-fat diet on firing rate of sympathetic nerves innervating brown adipose tissue in anesthetized rats. Physiol Behav 45: 1177–1182, 1989. [DOI] [PubMed] [Google Scholar]
- 26.Sell H, Berger JP, Samson P, Castriota G, Lalonde J, Deshaies Y, Richard D. Peroxisome proliferator-activated receptor gamma agonism increases the capacity for sympathetically mediated thermogenesis in lean and ob/ob mice. Endocrinology 145: 3925–3934, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Stocker SD, Meador R, Adams JM. Neurons of the rostral ventrolateral medulla contribute to obesity-induced hypertension in rats. Hypertension 49: 640–646, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Troy AE, Simmonds SS, Stocker SD, Browning KN. High fat diet attenuates glucose-dependent facilitation of 5-HT mediated responses in rat gastric vagal afferents. J Physiol 594: 99–114, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsukita S, Yamada T, Uno K, Takahashi K, Kaneko K, Ishigaki Y, Imai J, Hasegawa Y, Sawada S, Ishihara H, Oka Y, Katagiri H. Hepatic glucokinase modulates obesity predisposition by regulating BAT thermogenesis via neural signals. Cell Metab 16: 825–832, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Tupone D, Madden CJ, Morrison SF. Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat. J Neurosci 33: 14512–14525, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Vance KM, Rogers RC, Hermann GE. PAR1-activated astrocytes in the nucleus of the solitary tract stimulate adjacent neurons via NMDA receptors. J Neurosci 35: 776–785, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518–1525, 2009. [DOI] [PubMed] [Google Scholar]