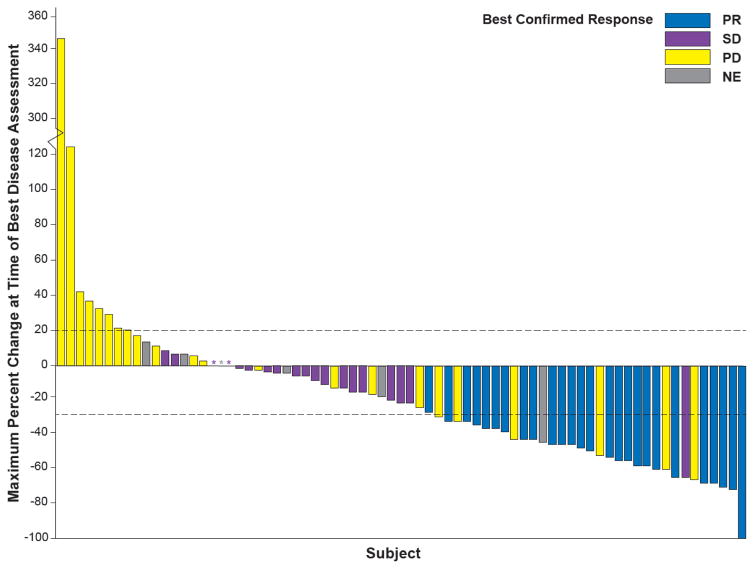
Figure 2. Response to dabrafenib in BRAF V600E–mutant NSCLC.
Maximum change in the sum of lesion diameters by best confirmed response in ≥ second-line patients treated with dabrafenib (N = 78) at the time of the clinical activity analyses. The dashed line at 20 represents the RECIST 1.1 definition for progressive disease, while the dashed line at −30 represents the definition for partial response. Asterisks represent patients with no change from baseline at the time of assessment. NE denotes not estimable, NSCLC non-small cell lung cancer, PD progressive disease, PR partial response, RECIST Response Evaluation Criteria In Solid Tumors, SD stable disease.