Abstract
Glioblastoma (GBM) is the most common primary malignant brain tumor in adults, with an average age of 64 years at the time of diagnosis. To study GBM, a number of mouse brain tumor models have been utilized. In these animal models, subjects tend to range from 6–12 weeks of age, which is analogous to that of a human teenager. Here, we examined the impact of age on host immunity and the gene expression associated with immune evasion in immunocompetent mice engrafted with syngeneic intracranial GL261. The data indicate that, in mice with brain tumors, youth conveys an advantage to survival. While age did not affect the tumor-infiltrating T cell phenotype or quantity, we discovered that old mice express higher levels of the immunoevasion enzyme, IDO1, which was decreased by the presence of brain tumor. Interestingly, other genes associated with promoting immunosuppression including CTLA-4, PD-L1 and FoxP3, were unaffected by age. These data highlight the possibility that IDO1 contributes to faster GBM outgrowth with advanced age, providing rationale for future investigation into immunotherapeutic targeting in the future.
Keywords: Cancer immunity, glioma, Treg, TDO, immunosuppression, tryptophan
INTRODUCTION
Glioblastoma (GBM) is the most common primary malignant brain tumor within the adult central nervous system (CNS). [1, 2] The median age of an adult patient diagnosed with GBM is 64 years old. [3] GBM is a highly aggressive brain tumor thought to arise by malignant transformation of glial precursor cells and/or mature glia. Despite maximal surgical resection, radiotherapy, and chemotherapy, overall survival (OS) remains at 14.6 months post-diagnosis with 26% of patients surviving two years. [4]
The dismal prognosis of GBM is, in part, due to the potently immunosuppressive brain tumor microenvironment. This is reflected by the accumulation of regulatory T cells (Tregs) [5, 6], myeloid derived suppressor cells (MDSC) [7, 8], IDO1 activity [9, 10], increased expression for immune checkpoint molecules, CTLA-4 and PD-1/PD-L1 [11–13] and immunosuppressive cytokine expression. [14–16] This is commensurate with a progressive impairment of the adaptive immune system with age. [17] Notably, CD8+ recent thymic emigrant levels are associated with the prognostic impact of age on clinical outcomes in GBM patients. [18]
The median age of an adult human GBM patient diagnosis is equivalent to ~74 weeks of age in mice. Given that the majority of brain tumor studies occur in relatively young mice, equivalent to 6–12 weeks old, we hypothesized that age may negatively impact the immune response to brain tumors. Here, we report that old immunocompetent mice engrafted syngeneic GL261 cell-based brain tumors have decreased survival when compared to young counterparts. Unexpectedly, there was no impact of age on the tumor-infiltrating T cell response. However, we found a substantial upregulation of mRNA for the immunosuppressive enzyme, IDO1, in old mouse brain when compared to young- or tumor bearing-brain. These data show for the first time that old mice with brain tumors have decreased survival and implicates IDO1 as a potential contributing factor that contributes to tumor growth.
MATERIALS AND METHODS
Mice and cell lines
Young (6-week old) and aged (72-week old) mice C57BL/6 (wild-type; Cat# 000664) were obtained from Jackson Laboratories and maintained in the Northwestern University Center for Comparative Medicine. Mice were intracranially injected at 8 or 74 weeks of age, respectively. All surgical procedures were completed in accordance with NIH guidelines on the care and use of laboratory animals. Mice were euthanized by injection of a lethal dose of Ketamine (200 mg/kg) and Xylazine (10 mg/kg) followed by cervical dislocation. GL261 cells were obtained from the NCI (Frederick, MD) and cultured in Dulbecco modified Eagle medium (DMEM) and supplemented with 10% fetal bovine serum (FBS), in addition to penicillin (100 μg/mL) and streptomycin (100 mg/mL) at 37°C. All products used for cell culture were purchased from Gibco Invitrogen.
Orthotopic intracranial injection model
Mice were anesthetized with 0.15 mL solution containing ketamine HCl (90 mg/mkg) and xylazine (10 mg/kg) with an intraperitoneal injection and administered meloxicam (2 mg/kg) through subcutaneous injection for pain management. The surgical site was shaved and prepared with iodine and 70% ethyl alcohol. A midline incision was made, followed by drilling a parietal burr hole 2 mm posterior to the coronal suture and 2 mm lateral to the sagittal suture. Mice were placed in a stereotactic frame and 2 × 105 GL261 cells in 2.5 μL saline were intracranially-injected (ic) with a 22-gauge needle at a depth of 3 mm. After needle removal, the skin was stapled.
Flow cytometry and T-cell stimulation
Brain tumor (BT), cervical lymph node (cLN) and spleen were isolated at 3 weeks post-intracranial injection (ic.) and mashed through a sterile 70 μm nylon mesh cell strainer (Fisher Scientific) with the plunger end of a 3 mL syringe into ice-cold DMEM. Single cell suspensions of BT were mixed in a PBS/30% Percoll solution and slowly pipetted onto a 70% Percoll cushion. Samples were centrifuged at 1200 × g for 30 minutes with no brake. The top layer was aspirated and the buffy coat, between the 30% and 70% percoll layers, was isolated and washed in cold PBS. Single cell suspensions of BT, cLN and spleen were then divided into three groups for staining: 1) unstimulated T cells, 2) stimulated T cells and 3) antigen-presenting cells. For stimulation, T cells were co-incubated with Cell Stimulation Cocktail (PMA/Ionomycin/Brefeldin A/Monensin; eBioscience) for 5 hours in DMEM at 37°C. Cells were incubated with antibodies in PBS + 2% FBS for 30 min on ice and according to Table 1. Samples were then permeabilized overnight at 4°C using Fix/Perm Buffer (eBioscience) and incubated accordingly. Cellular frequency was determined with an LSR Fortessa flow cytometer (BD) and Flowjo analysis software (TreeStar, Cupertino, CA).
Table 1. The experimental design for flow cytometric evaluation.
All antibody staining, except (*), was performed prior to overnight (o/n) permeablization. (*) indicates staining was performed after o/n permeablization.
| Group | Marker | Clone | Tag |
|---|---|---|---|
| 1 | anti-mouse CD3 | 145-2C11 | eFluor450 |
| anti-mouse CD4 | GK1.5 | PE-Cy7 | |
| anti-mouse CD8 | 53-6.7 | FITC | |
| anti-mouse/human CD44 | IM7 | APC | |
| anti-mouse/rat FoxP3 | FJK-16s | PE | |
| 2 | anti-mouse CD3 | 145-2C11 | PE-Cy7 |
| anti-mouse CD11b | M1/70 | FITC | |
| anti-mouse CD11c | N418 | PE | |
| anti-mouse CD45 | 30-F11 | eFluor450 | |
| anti-mouse Ly-6G (Gr1) | RB6-8C5 | APC | |
| 3 | anti-mouse CD3 | 145-2C11 | eFluor450 |
| anti-mouse CD4 | GK1.5 | PE-Cy7 | |
| anti-mouse CD8 | 53-6.7 | APC | |
| anti-mouse IFN-γ | XMG1.2 | FITC | |
| anti-mouse/rat IL-17A | eBio17B7 | PE |
HPLC analysis
HPLC analysis was carried out as previously described by Zhai, et al. [19].
Statistical analysis
Data are represented as the mean ±SEM. The statistical significance of the differences in mRNA expression and tumor-infiltrating T cell response was determined by two-tailed unpaired Student-t test for two groups and one-way ANOVA for groups of three or more followed by Turkey’s post-hoc test. Overall survival was defined as the time from implantations until death. Survival curves were plotted using the Kaplan-Meier method and compared by Gehan-Breslow-Wilcoxon test. Data were analyzed using Prism 6.0 software (GraphPad Software). A P value less than 0.05 was considered significant.
RESULTS
Impact of age on survival from brain tumors
To determine the impact of age on survival from malignant glioma, mice were intracranially-injected with GL261 cells at either 8 or 74 weeks of age and monitored for morbidity and/or mortality. As shown in Figure 1, young mice survive significantly longer than old mice, with a median OS of 27.5 days and 21.5 days, respectively (P=0.0292). These data confirm that advanced age is associated with decreased survival in response to brain tumor burden.
Figure 1. The overall survival of young and old mice with brain tumors.
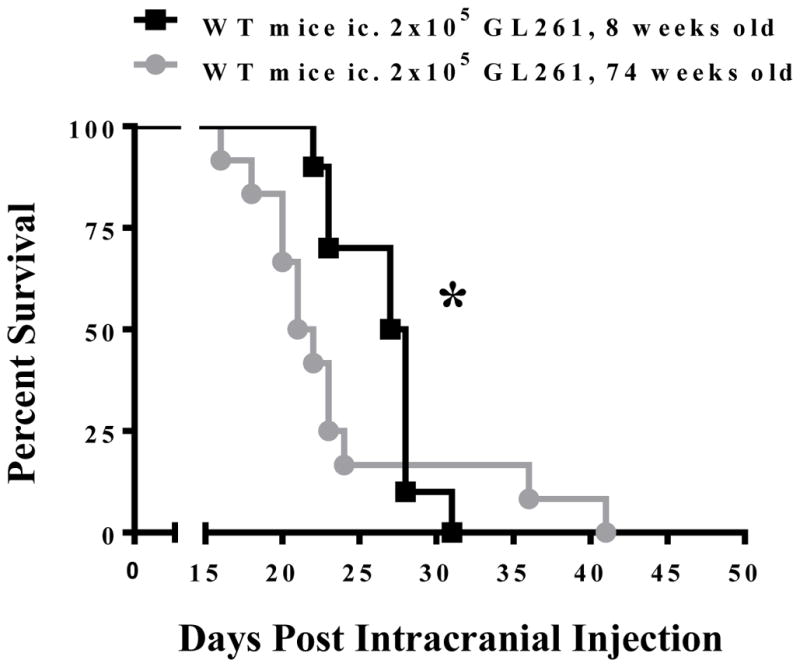
2 × 105 GL261 cells were intracranially-injected into 8 week old (black, n=10) or 74 week old (grey, n=12) C57BL/6 mice. The Kaplan-Meier curve represents mouse survival times over a time course of 50 days. * Gehan-Breslow-Wilcoxon test P = 0.0292.
Effect of age on genes that regulate brain tumor immunity
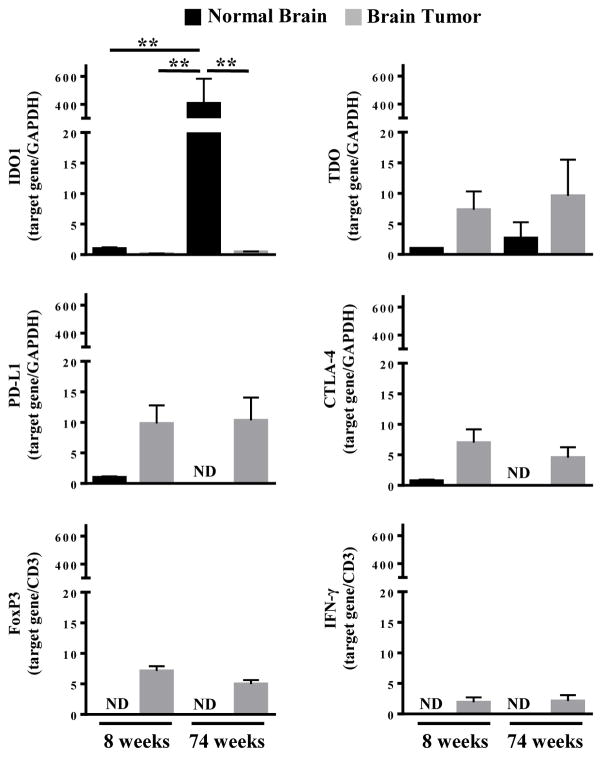
Given the association of older mice with the increased mortality rate due to brain tumor burden, we next examined the mRNA expression level for genes involved in regulating brain tumor immunity using the primers shown in Table 2. Brain tissue isolated from the contralateral hemisphere of the GL261 cell injection site served as a non-tumor control. Figure 2 demonstrates that IDO1 mRNA expression increases 406.4 fold in the old-, when compared to the young-brain (P=0.0018), which is congruent with previous observations. [20] However, there was no difference in IDO1 mRNA expression in brain tumors isolated from young and old mice, suggesting that the malignancy suppresses IDO1 levels in and/or around the tumor. In contrast to IDO1, neither age nor the presence of brain tumor affected the expression of TDO, CTLA-4, PD-L1, FoxP3 or IFNγ. Collectively, these data suggest that the high IDO1 level associated with advanced age, may contribute to a competitive advantage with respect to brain tumor growth.
Table 2. The experimental primers for PCR analysis of gene expression.
BioRad assay numbers are shown for all commercial primers.
| Gene | Primer |
|---|---|
| IDO1 (C-term3 mIDO1 Fwd) | Fwd: 5′-CGA GTG TGT GAA TGG TCT GGT-3′ Rev: 5′-TTG AGG GCT CTT CCG ACT TG-3′ |
| GAPDH | BioRad (Assay ID: qMmuCED0027497) |
| TDO | BioRad (Assay ID: qMmuCID0015202) |
| CD3e | BioRad (Assay ID: qMmuCID0027036) |
| PD-L1 | BioRad (Assay ID: qMmuCID0011922) |
| CTLA-4 | BioRad (Assay ID: qMmuCID0008808) |
| FoxP3 | BioRad (Assay ID: qMmuCID0022414) |
| IFN-gamma | BioRad (Assay ID: qMmuCID0006268) |
Figure 2. mRNA expression analysis for immunoregulatory genes in young and old mice with brain tumors.
RT-PCR quantitative analysis for IDO1, TDO, PD-L1, CTLA-4, FoxP3, and IFN-γ in GL261 cell-based glioma lysates isolated at 3 weeks post-intracranial engraftment in 8 week old (n=8) and 74 week old (n=5) WT mice. ND = Not detectable. Bar graphs shown as mean ± SEM. Significance determined by One-Way ANOVA followed by a Turkey’s post-hoc test (P<0.01).
Tryptophan (Trp) and kynurenine (Kyn) levels in brain tumors
We next set out to determine whether Trp and Kyn levels were changed between the young and old brain. However, the concentration of Trp and Kyn, along with the Kyn/Trp ratio, which is an indicator of IDO1 enzymatic activity, did not change in the serum or brain tumor as demonstrated in Figure 3. Taken together, the unchanged IDO1 mRNA expression in brain tumors isolated from young and old hosts, corresponds to a similar level of IDO1 activity.
Figure 3. Tryptophan (TRP) and kynurenine (KYN) levels in young and old mice with brain tumors.
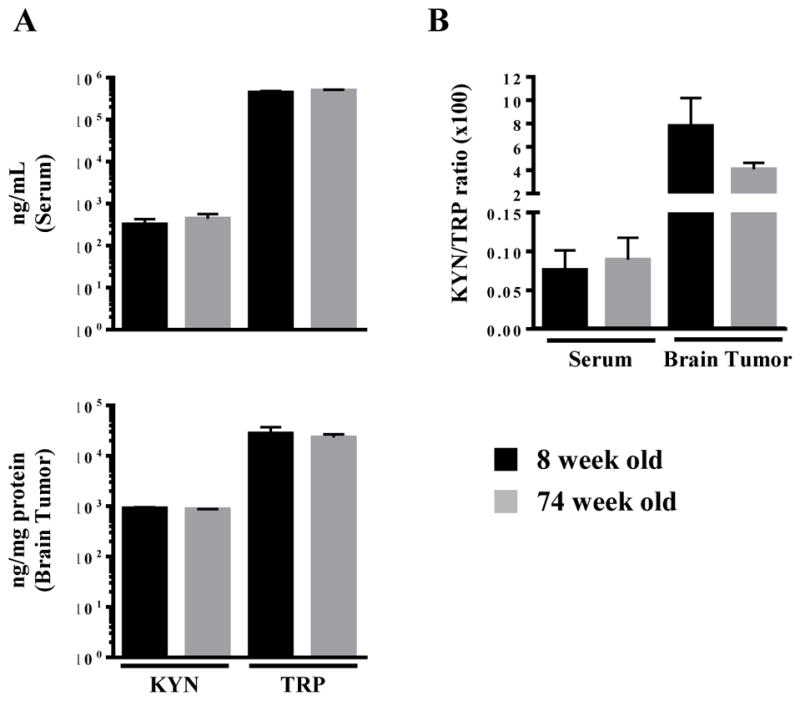
(A) Trp and Kyn levels in the serum and brain tumor (BT) were analyzed by HPLC in 8 week old (n=8) and 74 week old (n=5) mice at 3 weeks post-intracranial engraftment. There was no difference in the (B) KYN/TRP ratio. Bar graphs shown as mean ± SEM.
Impact of age on brain tumor-infiltrating leukocytes
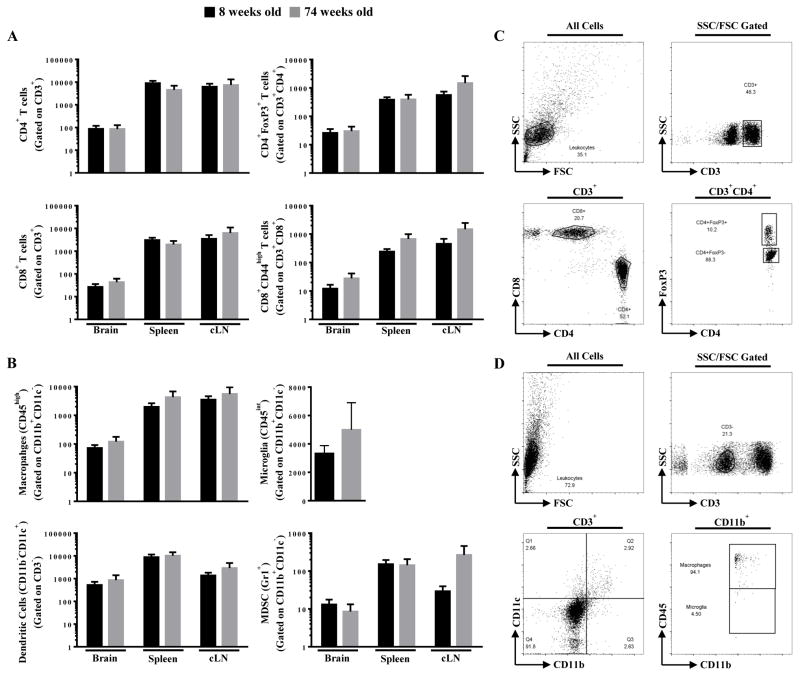
To determine if the effects of aging on survival was associated with immunological changes, T cells and antigen presenting cells were quantified and phenotyped in the brain tumor, cervical lymph nodes and spleen. However, age neither affected the quantity nor immune cell composition in tumor-bearing hosts (Figure 4). There were also no systemic differences in the number of T cells expressing the proinflammatory cytokines, IL-17A and IFN-γ, (Supplementary Figure 1). Taken together, age has no apparent impact on the local or system cellular immune response to a brain tumor in the parameters that we have measured.
Figure 4. T cell and antigen presenting cell (APC) levels in young and old mice with brain tumors.
Eight week old (n=8) and 74 week old (n=5) mice were intracranially-engrafted 2 × 105 GL261 cells. The absolute numbers of (A) CD4+ T cells, CD4+FoxP3+ T cells, CD8+ T cells, CD8+CD44high T cells, (B) macrophages, microglia, dendritic cells, and myeloid derived suppressor cells (MDSC) isolated from brain tumor, spleen, and cervical lymph node (cLN) of tumor-bearing mice at 3 weeks post-engraftment. The flow cytometry gating strategy is demonstrated for (C) T cell and (D) APC subpopulations is demonstrated. Bar graphs in A and B are shown as mean ± SEM.
DISCUSSION
Collectively, these data demonstrate that age negatively impacts survival in a syngeneic immunocompetent brain tumor model. It has also been demonstrated that IDO1 is expressed in 90–96% of glioblastoma [21, 22] and that tumor cell expression of IDO1 is critical for suppression of the antitumor immune response. [23] The data also confirm that, while IDO1 expression increases in the normal brain with age [24, 25], CNS tumors express similar IDO1 levels regardless of host age. Notably, there was a direct parallel between IDO1, Trp and Kyn levels between young and old mice with brain tumors.
A surprising finding from our investigation highlights a lack of difference in the cellularity of the immune response between young and old hosts with brain tumors. Previous studies have shown that the advanced age decreases the functionality of the immune response [26–28] and that immune cell infiltration is correlated to increased survival. [29] In GBM, the expression of immunosuppressive cytokines and accumulation of suppressor cells contributes to the potently immunosuppressive tumor microenvironment. However, we found no difference between the numbers of suppressive and/or proinflammatory T cells, when comparing young and aged mice with brain tumors. A possible factor that may be altered in this setting, but was not examined in this study, is recent thymic CD8+ T cell emigrants, which have been shown to differ with age in the GBM patient setting. [18]
A future direction arising from these studies is whether young and old brain tumor-bearing mice equally respond to- and/or tolerate immunotherapy, similarly. This is a clinically-relevant consideration given the number of immunotherapy-based clinical trials that have been deployed to treat adult GBM patients; many that present with advanced age. [30] Additionally, future work is also planned to address whether IDO1 blockade, prior to brain tumor engraftment, ameliorates the survival difference between young and old mice; which may be related, in part, to progenitor cell aging. [31] In total, these data highlight the need for a better understanding of the interaction and impact between age, immunity and brain tumors, which may facilitate more meaningful advances in the understanding and effectiveness of future immunotherapeutic approaches for GBM patients.
Supplementary Material
HIGHLIGHTS.
Advanced age decreases survival in an immunocompetent brain tumor model.
IDO1 expression is significantly higher in aged brain.
Age does not affect the phenotype or quantity of brain tumor infiltrating lymphocytes
Acknowledgments
Funding: C.D. James is supported by PHS grant numbers P50CA097257, R01CA159467, awarded by the NIH/NCI, as well as R01NS080619, awarded by the NIH/NINDS and D.A. Wainwright is supported by PHS grant numbers R00NS082381 and R01NS097851, awarded by the NIH/NINDS, U.S. Department of Health and Human Services; a Robert H. Lurie Comprehensive Cancer Center – Zell Scholar Program of the Zell Family Foundation Gift; and the Northwestern Brain Tumor Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ostrom QT, et al. Epidemiology of gliomas. Cancer Treat Res. 2015;163:1–14. doi: 10.1007/978-3-319-12048-5_1. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Ostrom QT, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.El Andaloussi A, Han Y, Lesniak MS. Prolongation of survival following depletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. J Neurosurg. 2006;105(3):430–7. doi: 10.3171/jns.2006.105.3.430. [DOI] [PubMed] [Google Scholar]
- 6.El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006;8(3):234–43. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues JC, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12(4):351–65. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raychaudhuri B, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13(6):591–9. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wainwright DA, et al. Recent developments on immunotherapy for brain cancer. Expert Opin Emerg Drugs. 2012;17(2):181–202. doi: 10.1517/14728214.2012.679929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uyttenhove C, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9(10):1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 11.Bloch O, et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19(12):3165–75. doi: 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsa AT, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–8. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 13.Fecci PE, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13(7):2158–67. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, et al. Increased transforming growth factor-beta2 in epidermal growth factor receptor variant III-positive glioblastoma. J Clin Neurosci. 2011;18(6):821–6. doi: 10.1016/j.jocn.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Huettner C, Paulus W, Roggendorf W. Messenger RNA expression of the immunosuppressive cytokine IL-10 in human gliomas. Am J Pathol. 1995;146(2):317–22. [PMC free article] [PubMed] [Google Scholar]
- 16.Nitta T, et al. Selective expression of interleukin-10 gene within glioblastoma multiforme. Brain Res. 1994;649(1–2):122–8. doi: 10.1016/0006-8993(94)91055-3. [DOI] [PubMed] [Google Scholar]
- 17.Miller JP, Allman D. Linking age-related defects in B lymphopoiesis to the aging of hematopoietic stem cells. Semin Immunol. 2005;17(5):321–9. doi: 10.1016/j.smim.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Wheeler CJ, et al. Thymic CD8+ T cell production strongly influences tumor antigen recognition and age-dependent glioma mortality. J Immunol. 2003;171(9):4927–33. doi: 10.4049/jimmunol.171.9.4927. [DOI] [PubMed] [Google Scholar]
- 19.Zhai L, et al. The kynurenine to tryptophan ratio as a prognostic tool for glioblastoma patients enrolling in immunotherapy. J Clin Neurosci. 2015;22(12):1964–8. doi: 10.1016/j.jocn.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braidy N, et al. Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. FEBS J. 2011;278(22):4425–34. doi: 10.1111/j.1742-4658.2011.08366.x. [DOI] [PubMed] [Google Scholar]
- 21.Uyttenhove C, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9(10):1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 22.Mitsuka K, et al. Expression of indoleamine 2,3-dioxygenase and correlation with pathological malignancy in gliomas. Neurosurgery. 2013;72(6):1031–8. doi: 10.1227/NEU.0b013e31828cf945. discussion 1038–9. [DOI] [PubMed] [Google Scholar]
- 23.Humphries W, et al. The role of tregs in glioma-mediated immunosuppression: potential target for intervention. Neurosurg Clin N Am. 2010;21(1):125–37. doi: 10.1016/j.nec.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pertovaara M, et al. Indoleamine 2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech Ageing Dev. 2006;127(5):497–9. doi: 10.1016/j.mad.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Marttila S, et al. Aging-associated increase in indoleamine 2,3-dioxygenase (IDO) activity appears to be unrelated to the transcription of the IDO1 or IDO2 genes in peripheral blood mononuclear cells. Immun Ageing. 2011;8:9. doi: 10.1186/1742-4933-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fulop T, et al. Potential role of immunosenescence in cancer development. Ann N Y Acad Sci. 2010;1197:158–65. doi: 10.1111/j.1749-6632.2009.05370.x. [DOI] [PubMed] [Google Scholar]
- 27.Hakim FT, et al. Aging, immunity and cancer. Curr Opin Immunol. 2004;16(2):151–6. doi: 10.1016/j.coi.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007;70(3):179–89. doi: 10.1111/j.1399-0039.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 29.Kmiecik J, et al. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol. 2013;264(1–2):71–83. doi: 10.1016/j.jneuroim.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Wainwright DA, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20(20):5290–301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikheev AM, et al. Increased age of transformed mouse neural progenitor/stem cells recapitulates age-dependent clinical features of human glioma malignancy. Aging Cell. 2012;11(6):1027–35. doi: 10.1111/acel.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.