Integration of research into a comprehensive strategy for tuberculosis elimination is essential. Engaging the full spectrum of the tuberculosis community is needed to develop game-changing research and implementation strategies.
Keywords: tuberculosis, elimination, biomedical, research, global health
Abstract
Tuberculosis has impacted human health for millennia. The World Health Organization estimated that, in 2014, 9.6 million people developed tuberculosis and 1.5 million people died from the disease. In May 2014, the World Health Assembly endorsed the new “End TB Strategy” that presents a pathway to tuberculosis elimination. The strategy outlines 3 areas of emphasis, one of which is intensified research and innovation. In this article we highlight the essential role for fundamental tuberculosis research in the future of tuberculosis diagnostics, treatment, and prevention. To maximize the impact of fundamental research, we must foster collaboration among all stakeholders engaged in tuberculosis research and control to facilitate open dialogue to assure that critical gaps in outcome-oriented science are identified and addressed. We present here a framework for future discussions among scientists, physicians, research and development specialists, and public health managers for the reinforcement of national and international strategies toward tuberculosis elimination.
“The field of TB research has suffered enormous neglect over the past several decades and the consequences have been striking … It is critical to question the usual assumptions that have driven the field of TB research, and think in new and innovative ways, employing all the modern tools of biomedical research. Only by doing so can we develop the transforming innovations that are needed to end the global TB pandemic.”
— Dr. Anthony Fauci Director of the US National Institute of Allergy and Infectious Diseases [1]
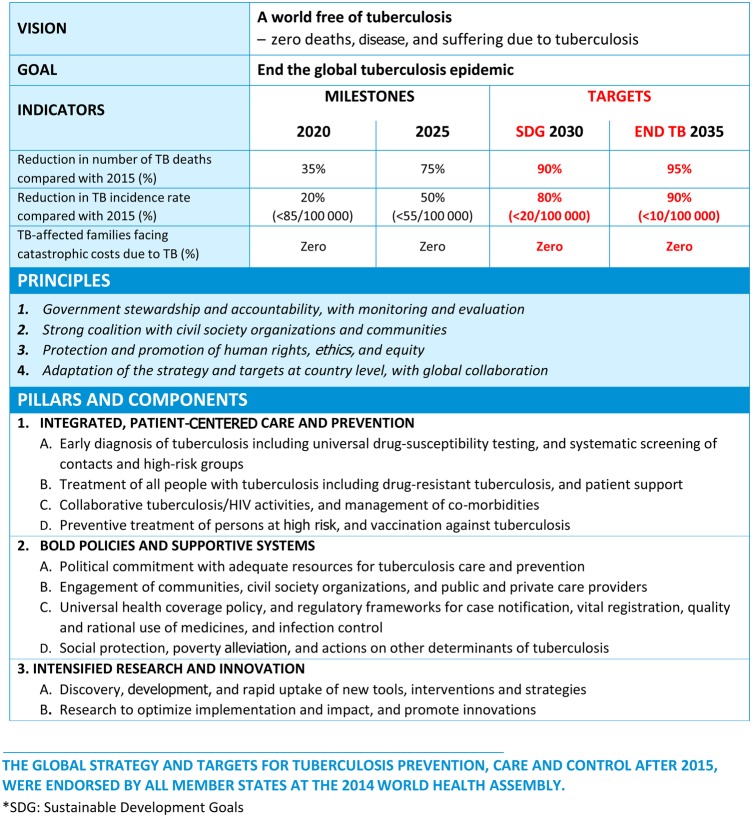
Tuberculosis has plagued humankind since its earliest existence [2, 3], and the rise of drug-resistant tuberculosis threatens to take us back to the preantibiotic era [4]. Revolutionary new technology and better ways to provide patient care are imperative to achieve tuberculosis elimination. For the first time, the recently adopted World Health Organization (WHO) “End TB Strategy 2016–2035” [5] recognizes the need “to promote the research and knowledge-generation required to end the global tuberculosis epidemic and eliminate tuberculosis” as one of the 3 fundamental pillars for global action: (1) integrated patient-centered care and prevention; (2) bold policies and supportive systems; and (3) intensified research and innovation (Figure 1).
Figure 1.
The World Health Organization's new “End TB Strategy.” Abbreviations: HIV, human immunodeficiency virus; TB, tuberculosis.
Elimination of tuberculosis is an ambitious goal. The WHO recently reported that 9.6 million people developed tuberculosis worldwide in 2014, and 1.5 million died from the disease, including 400 000 individuals who were also infected with human immunodeficiency virus (HIV) [6, 7]. Moreover, an estimated 480 000 of those tuberculosis patients had the multidrug-resistant (MDR) form, and 190 000 individuals died from MDR tuberculosis. Around one-third of persons with MDR tuberculosis received treatment and only 50% of those were successfully treated [6, 8]. Any realistic prospect of eliminating tuberculosis would rely on the development of innovative diagnostic, treatment, and prevention tools coupled with improved uptake of existing technologies [9]. The “research” pillar thus advocates for the development of public health outcome–oriented research that can drastically alter the tuberculosis control and care landscape through the discovery of new tools and interventions, and their optimal uptake [10].
To accomplish this, dialogue between all stakeholders in tuberculosis (biomedical scientists, medical officers, healthcare workers, public health experts, and program managers) will be needed to define the tuberculosis prevention and care needs that can only be addressed through intensified research.
This article outlines a vision of how the continuum of research aimed at responding to increased needs for tuberculosis control can be integrated through a fundamental research approach. We refer here to “fundamental research” to designate all scientific studies that are conducted as part of basic, translational, clinical, and epidemiological research that contribute to the understanding of tuberculosis and describe its underlying biological and medical mechanisms. Fundamental research thus spans several disciplines of science and can serve to connect these disciplines through cross-functional, integrated projects. Furthermore, we refer to “translational research” as all scientific studies that contribute directly to the development of new diagnostics, drugs, and vaccines, as well as to diagnostics, treatment, and prevention strategies. The intent of this article is to present a framework for future discussions among scientists, physicians, research and development specialists, and operational researchers for the development of leading strategies toward tuberculosis elimination.
FUNDAMENTAL TUBERCULOSIS RESEARCH: OVERVIEW AND CONTRIBUTIONS
Several aspects of fundamental tuberculosis research strive to understand the biology of Mycobacterium tuberculosis (Mtb) and its interaction with the human host. These are comprised of interconnected, complementary areas that require continued emphasis and support to refine our understanding of human tuberculosis globally: How does Mtb cause disease? What are the pathways that allow the pathogen to remain dormant or evade the immune system? How can one identify biological markers that characterize the various stages of infection and disease? Indeed, fundamental scientific discoveries provide the biological knowledge that underlies all product development and new technologies that are eventually translated into clinical application. For example, the recently developed Xpert MTB/RIF diagnostic assay is built upon an understanding of the genetic basis of rifampin resistance in Mtb [11] and makes use of molecular assays that were developed as research tools then integrated into a diagnostic platform. Without fundamental research, none of the underlying knowledge that drives this effective diagnostic tool would be available.
The number of research questions being addressed by scientists studying basic biology is vast and can be referenced in the recently developed International Roadmap for Tuberculosis Research [12]. A key issue for the integration of fundamental science into a larger elimination strategy is to find iterative connections between basic science and implementation, which will require discussion among scientific disciplines that do not usually interact or share a common “language.” We present here some examples to illustrate which areas of research may benefit from broader engagement and discussion (Figure 2).
Figure 2.
International Roadmap for Tuberculosis Research [12]: priorities in fundamental research for tuberculosis. Abbreviation: TB, tuberculosis.
Understanding the biology of Mtb—the function of its intracellular machinery and the biochemical pathways critical for its survival, pathogenesis, and development of drug resistance—is essential for developing control measures. Remarkable progress has been taking place recently in the identification of (1) specific bacterial processes against which new therapeutics can be developed [13–15]; (2) host or pathogen molecules and pathways that are involved in various stages of infection and disease and that can be translated into new diagnostic tools [16]; and (3) genetic markers of microbial drug resistance that can help the development of more rapid methods of identifying MDR/extremely drug-resistant tuberculosis [17].
Opportunities for cross-disciplinary dialogue also exist in the area of animal studies that are used to identify product candidates for later clinical development [18]. While it must be recognized that animal models are used to create hypotheses for basic science to better understand infection and disease, models are also used to simulate host/pathogen dynamics against which candidate vaccines and therapeutics are directed, or which serve to diagnose infection or disease [19]. However, a thorough understanding of disease dynamics in humans is still lacking, and many models are created based on what is measureable in animals, which can create statistically significant outcomes but may have limited biological relevance for affecting human outcomes [20]. To reach this goal, opportunities must be created for physicians, animal modelers, and basic scientists to discuss key issues in tuberculosis pathology to arrive at endpoints that are clinically meaningful, can be measured in animals, and can provide the necessary perspectives to better understand the realities, limitations, and predictive power of each other's scientific approaches. An example would be the role of measuring bacterial organ burden in animals as an indicator of treatment or prevention success. In humans this endpoint can only be measured in sputum and likely underreports the extent of persistent infection that may drive disease relapse. The iterative process of reevaluating both animal and human clinical trial data can radically help to refine the predictive power of animal models.
INTEGRATION OF FUNDAMENTAL RESEARCH INTO GLOBAL TUBERCULOSIS ELIMINATION STRATEGIES: CURRENT CHALLENGES AND OPPORTUNITIES
Fundamental research holds promise for modernization of interventions against tuberculosis; however, it is not always clear to both scientists and the wider tuberculosis control community how research directly impacts elimination efforts.
Indeed, the difficulty in connecting basic scientific studies with tangible outcomes that benefit patients, clinicians, and program managers has historically prevented interactions between the most basic and most applied disciplines of research. It has to be recognized that to innovate in tuberculosis research, fundamental research for the generation of knowledge must continue to be supported, even in the absence of an immediate opportunity of translating this knowledge into interventions. However, and most important, investigators studying processes with the potential to inform product development would greatly benefit from a thorough understanding of the realities of tuberculosis patient care and all involved logistics and decision algorithms, as well as general practices and expectations for developing clinical candidates of diagnostics, drugs, and vaccines. Exposing basic research scientists to the realities of tuberculosis control in endemic settings would allow better understanding of the challenges and realities of tuberculosis patient care and would provide a valuable opportunity to create a deeper sense of where tuberculosis research needs to be applied and create connections between basic and clinical/operational disciplines.
For fundamental research to contribute effectively to tuberculosis control and patient care, it is critical that operational aspects of tuberculosis care are understood by all, so that scientific findings can be combined with appropriate technologies to tailor new diagnostics, drugs, and vaccines to the setting in which they may be used [21]. Some examples of key tuberculosis control questions and the ways in which advances in fundamental research can contribute are discussed below.
Preventing Tuberculosis
Prevention of tuberculosis can be addressed in multiple ways: preventing transmission and Mtb infection, and/or progression to active tuberculosis [22]. Although it is currently not well understood what factors underlie transmission of Mtb and why certain patients are more likely to transmit the bacilli than others and therefore disproportionally contribute to the spread of tuberculosis, innovative new models are being developed to study transmission of Mtb directly from host to host rather than relying on artificial infection [23, 24]. Results from recent clinical trials of advanced vaccine candidates have again highlighted the need to better understand the reason why exposed persons diverge so widely in their capacity to control infection and how confounding factors such as age, sex, immune status, comorbidities, and coinfections contribute to these differing responses. Several cohort studies that are combining state-of-the-art immunology, microbiology, and genetics techniques promise to give better insight into the ability of the immune system to control Mtb infection, define more biologically relevant immune markers to be elicited by vaccines, define what vaccination approaches may be optimal for what age and risk groups, and thus contribute to the development of new vaccines and chemoprophylactic approaches [25–28]. To better understand the epidemiology, spread, and ability of specific strains of Mtb to cause active tuberculosis, large-scale sequencing efforts as well as detailed molecular dissections of Mtb virulence factors are defining the genetic background, and resulting biochemical characteristics of the pathogen may contribute to the development of new diagnostic tests [29–31]. Fundamental research, utilizing state-of-the-art laboratory and bioinformatics approaches, thus has the potential to explain the molecular basis of these biological phenomena, particularly when conducted in conjunction with clinical studies and clinical trials of vaccine candidates in a variety of epidemiological settings to estimate their efficacy and also to learn about factors that may interfere with their ability to prevent tuberculosis [26, 32].
Diagnosing Tuberculosis
Early diagnosis of drug-sensitive and drug-resistant disease is an important component of the global tuberculosis strategy. The current diagnostic methods are most applicable to patients with active, transmissible tuberculosis. However, the detection of the presence of Mtb before active disease commences is much more difficult and relies on the measurement of markers that can indirectly point to the presence of live organisms [33]. The search for biomarkers and biosignatures through integration of sophisticated molecular analysis of diverse clinical specimens, combined with in silico and bioinformatics models, is starting to bear fruit and deliver combinations of immune and other biochemical markers that may allow identification of high-risk individuals [27]. Similarly, advances in the development of more sensitive tests and technologies for diagnosis of drug-sensitive and drug-resistant tuberculosis from adult and pediatric specimens have the potential to transform how tuberculosis is detected at the point of care [34].
Treating Tuberculosis
The currently recommended tuberculosis treatment regimens have notable limitations. They are associated with side effects, and due to a duration of treatment that typically lasts at least 6 months, place a significant burden on the patients and the health systems [35]. In addition, patients' inability to complete the standard treatment regimen or take adequate doses of the medications favors the emergence of drug-resistant strains that are much more difficult to treat. To improve treatment of tuberculosis, parallel efforts are needed in basic and translational research to develop new drug candidates, regimens, and treatment options and in programmatic research to improve access to and delivery of quality-controlled first- and second-line drugs. The development of specific “target product profiles” for new tuberculosis drugs and tuberculosis treatment regimens can assist in aligning the needs of end users with the targets and specifications that developers should meet for the performance and operational characteristics of new tuberculosis treatment regimens [36].
Recent trials of shortened tuberculosis treatment provide some insight into why it is not currently possible to treat tuberculosis with only several weeks of chemotherapy, as is the case for other common bacterial infections [37–39]. New hypotheses are emerging that point to the importance of drug distribution into lesions and key organs to affect diverse subpopulations of Mtb that are difficult to eradicate and may contribute to the long duration of treatment [40, 41]. Similarly, new bacterial targets against which drugs could be developed are emerging [15]. These studies are facilitated through the close collaboration among physicians, pathologists, and researchers, and guide the rational approach to identification of acceptable new treatment options.
INTEGRATING FUNDAMENTAL RESEARCH IN TUBERCULOSIS CONTROL: FORMING UNIFIED TEAMS
Designing and conducting health outcome–oriented biomedical research projects present both unique opportunities and challenges for scientists, clinicians, industry, and funders alike. The potential contributions of fundamental research to tuberculosis diagnosis, prevention, and treatment described above involve the integration of multiple distinct fields of research that traditionally do not intersect. As recommended in the WHO Global Framework for Tuberculosis Research [10], cross-disciplinary research, in close collaboration with tuberculosis control programs and governments, has the potential for improving tuberculosis care. For instance, operational and implementation research studies conducted by medical researchers in collaboration with tuberculosis control programs are essential for mutual information exchange, to inform public health recommendations and create concrete medical evidence upon which global care recommendations can be based [42]. A first step in the formation of unified teams could be through symbiotically sharing data—going beyond putting research data into the open access space, by reaching out to discuss and share findings with partners in the field and working together to translate research into the clinic as well as move the field into new frontiers [43].
Funding agencies supporting global tuberculosis research each have a unique mission and funding process. For cross-disciplinary, integrated research to be successful, it must be recognized that co-funding of large projects remains challenging. Because broad, cross-disciplinary projects span the mission of multiple funders [44], projects need to be designed so that the involved disciplines can seek funding from respective agencies that support their very area of research. To assist researchers in targeting their project components to the most appropriate funding mechanism, we envision complementing existing public information about funding opportunities with more detailed and nuanced information about the respective funders through the development of a targeted tuberculosis research “Funders' Compendium” [10]. Furthermore, a “Funders' Forum” has been advocated and is being established by WHO to facilitate information exchange to help guide grant seekers, as well as to discuss ongoing research portfolios and identify gaps in common areas of science [10].
FUNDING OF FUNDAMENTAL TUBERCULOSIS RESEARCH
For the past 5 years, global funding for tuberculosis research and development has remained flat and well below the estimated amount needed to efficiently address global control of the epidemic [45]. A major part of the global research funding for tuberculosis is provided by only a few agencies, all of which operate under specific missions and funding principles. For example, within the US government's National Institutes of Health (NIH), the National Institute of Allergy and Infectious Diseases is one of the largest supporters of basic, applied, and clinical research in tuberculosis and HIV/tuberculosis [46]. The NIH, together with the Bill & Melinda Gates Foundation, the European Commission, the UK Department for International Development, and the Wellcome Trust, as well as some pharmaceutical companies conducting clinical trials, currently make up the top tier of supporters for tuberculosis biomedical and product development research. However, whereas most of the top 10 tuberculosis funding bodies are found in the United States and Europe [45], the burden of tuberculosis continues to be felt primarily in middle- and low-income nations. As countries transition into prosperity, it is necessary that they commit to the fight against tuberculosis through contributions to public health and the establishment of resources for fundamental research.
MAXIMIZING IMPACT
To combat this old disease, reinvigorated intellectual input and action by all stakeholders—from basic researchers to medical professionals and advocates—is required to arrive at new strategies toward elimination. The new WHO End TB Strategy highlights the critical importance of biomedical research as an integral part of a global strategy to governments and public health officials and emphasizes that, in the 21st century, research can and must complement and inform traditional control strategies. Integrated research efforts that are focused on key issues with the greatest potential to lead to a reduction in incidence, prevalence, and death can and should be prioritized for action. The only way to obtain the necessary “quantum leap” in tuberculosis research is to work together to improve the foundation of knowledge that is needed to understand the biology of human tuberculosis and firm up the foundation for new diagnostic, treatment, and prevention strategies.
To maximize impact, the tuberculosis research and control community must engage in a continuous and open dialogue to assure that critical gaps in outcome-oriented science are identified and addressed with minimal duplication of effort. Basic researchers are discovering the knowledge needed to create new scientific tools, diagnostics, therapeutics, and vaccines. Translational scientists can provide crucial information about the feasibility of transforming scientific findings into products. Last, clinicians have vital knowledge about the application of tuberculosis care. By working together as members of larger cross-disciplinary teams, we have the opportunity to shape this field and change the course of, and ultimately end, tuberculosis in humans.
Notes
Author contributions. All authors contributed equally to this work.
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fauci A. Fighting TB should be priority. Available at: http://www.nbcnews.com/id/33890464/ns/health-infectious_diseases/%20%28accessed%2027%20January%202011%29#.Ur3a8ltDsVw Accessed 19 April 2016.
- 2.Zink AR, Sola C, Reischl U et al. . Characterization of Mycobacterium tuberculosis complex DNAs from Egyptian mummies by spoligotyping. J Clin Microbiol 2003; 41:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Comas I, Gagneux S. The past and future of tuberculosis research. PLoS Pathog 2009; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. CDC's top ten: 5 health achievements in 2013 and 5 health threats in 2014. Available at: http://blogs.cdc.gov/cdcworksforyou24-7/2013/12/cdc%E2%80%99s-top-ten-5-health-achievements-in-2013-and-5-health-threats-in-2014/ Accessed 19 April 2016.
- 5.World Health Organization. Global strategy and targets for tuberculosis prevention, care and control after 2015. WHO/HTM/TB/2015.19. Geneva, Switzerland: WHO. Available at: http://www.who.int/tb/End_TB_brochure.pdf?ua=1. Accessed 16 April 2016.
- 6.World Health Organization. Global tuberculosis report 2015. WHO/HTM/TB/2015.22. Geneva, Switzerland: WHO, 2015. Available at: http://www.who.int/tb/publications/global_report/en/ Accessed 19 April 2016. [Google Scholar]
- 7.World Health Organization. Global tuberculosis report. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 8.World Health Organization. Tuberculosis 2015 fact sheet Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 9.Lienhardt C, Espinal M, Pai M, Maher D, Raviglione MC. What research is needed to stop TB? Introducing the TB Research Movement. PLoS Med 2011; 8:e1001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Global action framework for TB research in support of the third pillar of WHO's End TB Strategy. WHO/HTM/TB/2015.26. Geneva, Switzerland: WHO, 2015. Available at: http://www.who.int/tb/features_archive/global_framework_research/en/ Accessed 19 April 2016.
- 11.Boehme CC, Nabeta P, Hillemann D et al. . Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010; 363:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization and Stop TB Partnership. An international roadmap for tuberculosis research. Geneva, Switzerland: WHO, 2011. Available at: http://www.stoptb.org/assets/documents/resources/publications/technical/tbresearchroadmap.pdf13 Accessed 19 April 2016. [Google Scholar]
- 13.Rhee KY, de Carvalho LP, Bryk R et al. . Central carbon metabolism in Mycobacterium tuberculosis: an unexpected frontier. Trends Microbiol 2011; 19:307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ioerger TR, O'Malley T, Liao R et al. . Identification of new drug targets and resistance mechanisms in Mycobacterium tuberculosis. PLoS One 2013; 8:e75245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chim N, Habel JE, Johnston JM et al. . The TB Structural Genomics Consortium: a decade of progress. Tuberculosis (Edinb) 2011; 91:155–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blankley S, Berry MPR, Graham CM, Bloom CI, Lipman M, O'Garra A. The application of transcriptional blood signatures to enhance our understanding of the host response to infection: the example of tuberculosis. Phil Trans R Soc B 2014; 369:20130427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller B, Borrell S, Rose G, Gagneux S. The heterogeneous evolution of multidrug-resistant Mycobacterium tuberculosis. Trends Genet 2013; 29:160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassidy JP, Martineau AR. Innate resistance to tuberculosis in man, cattle and laboratory animal models: nipping disease in the bud? J Comp Pathol 2014; 151:291–308. [DOI] [PubMed] [Google Scholar]
- 19.Kaushal D, Mehra S, Didier PJ, Lackner AA. The non-human primate model of tuberculosis. J Med Primatol 2012; 41:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nuermberger E, Sizemore C, Romero K, Hanna D. Toward an evidence-based nonclinical road map for evaluating the efficacy of new tuberculosis (TB) drug regimens: proceedings of a critical path to TB drug regimens—National Institute of Allergy and Infectious Diseases In Vivo Pharmacology Workshop for TB Drug Development. Antimicrob Agents Chemother 2016; 60:1177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lienhardt C. Fundamental research is the key to eliminating TB. Nature 2014; 507:401. [DOI] [PubMed] [Google Scholar]
- 22.Mascart F, Locht C. Integrating knowledge of Mycobacterium tuberculosis pathogenesis for the design of better vaccines. Expert Rev Vaccines 2015; 14:1573–85. [DOI] [PubMed] [Google Scholar]
- 23.Stein RA. Super-spreaders in infectious diseases. Int J Infect Dis 2011; 15:e510–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dharmadhikari AS, Basaraba RJ, Van Der Walt ML et al. . Natural infection of guinea pigs exposed to patients with highly drug-resistant tuberculosis. Tuberculosis (Edinb) 2011; 91:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young DB, Gideon HP, Wilkinson RJ. Eliminating latent tuberculosis. Trends Microbiol 2009; 17:183–8. [DOI] [PubMed] [Google Scholar]
- 26.Brennan MJ, Thole J. Tuberculosis vaccines: a strategic blueprint for the next decade. Tuberculosis (Edinb) 2012; 92(suppl 1):S6–13. [DOI] [PubMed] [Google Scholar]
- 27.Zak DE, Penn-Nicholson A, Scriba TJ et al. . A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet 2016; doi:10.1016/S0140-6736(15)01316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahapatra S, Hess AM, Johnson JL et al. . A metabolic biosignature of early response to anti-tuberculosis treatment. BMC Infect Dis 2014; 14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farhat MR, Sultana R, Iartchouk O et al. . Genetic determinants of drug resistance in Mycobacterium tuberculosis and their diagnostic value. Am J Respir Crit Care Med 2016; doi:10.1164/rccm.201510-2091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ssengooba W, Meehan CJ, Lukoye D et al. . Whole genome sequencing to complement tuberculosis drug resistance surveys in Uganda. Infect Genet Evol 2016; 40:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehrt S, Rhee K, Schnappinger D. Mycobacterial genes essential for the pathogen's survival in the host. Immunol Rev 2015; 264:319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mangtani P, Abubakar I, Ariti C et al. . Protection by BCG against tuberculosis: a systematic review of randomised controlled trials. Clin Infect Dis 2014; 58:470–80. [DOI] [PubMed] [Google Scholar]
- 33.Esmail H, Barry CE, Wilkinson RJ. Understanding latent tuberculosis: the key to improved diagnostic and novel treatment strategies. Drug Discov Today 2012; 17:514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haas CT, Roe JK, Pollara G, Mehta M, Noursadeghi M. Diagnostic ‘omics’ for active tuberculosis. BMC Med 2016; 14:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. Treatment of tuberculosis: guidelines—4th ed. Geneva, Switzerland: WHO, 2010. [PubMed]
- 36.Lienhardt C, Raviglione M, Spigelman M et al. . New drugs for the treatment of tuberculosis: needs, challenges, promise, and prospects for the future. J Infect Dis 2012; 205(suppl 2):S241–9. [DOI] [PubMed] [Google Scholar]
- 37.Merle CS, Fielding K, Sow OB et al. . A four-month gatifloxacin-containing regimen for treating tuberculosis. N Engl J Med 2014; 371:1588–98. [DOI] [PubMed] [Google Scholar]
- 38.Jindani A, Harrison TS, Nunn AJ et al. . High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. N Engl J Med 2014; 371:1599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gillespie SH, Crook AM, McHugh TD et al. . Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 2014; 371:1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prideaux B, Via LE, Zimmerman MD et al. . The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat Med 2015; 21:1223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nathan C, Barry CE. TB drug development: immunology at the table. Immunol Rev 2015; 264:308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knight GM, Dharan NJ, Fox GJ et al. . Bridging the gap between evidence and policy for infectious diseases: how models can aid public health decision-making. Intern J Infect Dis 2016; 42:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Longo DL, Drazen JM. Data sharing. N Engl J Med 2016; 374:276–7. [DOI] [PubMed] [Google Scholar]
- 44.Stop TB Partnership. The global plan to Stop TB 2011–2015. Available at: http://www.stoptb.org/assets/documents/global/plan/TB_GlobalPlanToStopTB2011-2015.pdf Accessed 19 April 2016.
- 45.Treatment Action Group; Frick M. Report on tuberculosis research funding trends, 2005–2013. Available at: http://www.treatmentactiongroup.org/sites/g/files/g450272/f/201505/TAG_2014_TB_Funding_Report_2nd_Ed.pdf. Accessed 19 April 2016.
- 46.National Institute of Allergy and Infectious Diseases Tuberculosis Working Group. NIAID research agenda: multidrug-resistant and extensively drug-resistant tuberculosis, 2007. Available at: http://www.niaid.nih.gov/topics/tuberculosis/research/documents/mdrxdrresearchagenda.pdf. Accessed 19 April 2016.