Abstract
The neural balance between facilitation and inhibition determines the final tendency of central sensitization. Nerve injury-induced hypersensitivity was considered as the results from the enhanced ascending facilitation and the diminished descending inhibition. The role of dopaminergic transmission in the descending inhibition has been well documented, but its underlying molecular mechanisms are unclear. Previous studies demonstrated that the lysine dimethyltransferase G9a/G9a-like protein (Glp) complex plays a critical role in cocaine-induced central plasticity, and given cocaine’s role in the nerve system is relied on its function on dopamine system, we herein proposed that the reduced inhibition of dopaminergic transmission was from the downregulation of tyrosine hydroxylase expression by G9a/Glp complex through methylating its gene Th. After approval by the Animal Care and Use Committee, C57BL/6 mice were used for pain behavior using von Frey after spared nerve injury, and Th CpG islands methylation was measured using bisulfite sequencing at different nerve areas. The inhibitor of G9a/Glp, BIX 01294, was administered intraventricularly daily with bolus injection. The protein levels of G9a, Glp, and tyrosine hydroxylase were measured with immunoblotting. Dopamine levels were detected using high-performance liquid chromatography. The expression of G9a but not Glp was upregulated in ventral tegmental area at post-injury day 4 till day 49 (the last day of the behavioral test). Correspondingly, the Th CpG methylation is increased, but the tyrosine hydroxylase expression was downregulated and the dopamine level was decreased. After the intracerebroventriclar injection of BIX 01294 since the post-injury days 7 and 14 for consecutive three days, three weeks, and six weeks, the expression of tyrosine hydroxylase was upregulated with a significant decrease in Th methylation and increase in dopamine level. Moreover, the pain after G9a/Glp inhibitor was attenuated significantly. In sum, methytransferase G9a/Glp complex partially controls dopaminergic transmission by methylating Th in peripheral nerve injury-induced neuropathic pain.
Keywords: Nerve injury, tyrosine hydroxylase, G9a/Glp, dopamine, desensitization
Introduction
Nerve injury-induced neuropathic pain remains a severe and prevalent problem that can reduce quality of life and cause prolonged suffering in patients, affecting a large group of individuals. Although we have made many advances in understanding the pathogenesis of neuropathic pain with the development of new analgesic techniques and agents, it still remains incompletely controlled largely due to our insufficient knowledge of its underlying mechanisms.1,2
The final tendency of central sensitization is determined by the neural balance between facilitation and inhibition and the susceptibility to pain is supposed to depend on the balance of ascending and descending signaling pathways in pain.1,3,4 Nerve injury-induced pain hypersensitivity was considered as the results from the enhanced ascending facilitation and the diminished descending inhibition. The role of dopaminergic transmission in the descending pain inhibitory system has been well documented and dysregulations in dopaminergic transmission may contribute to the altered pain processing,5–7 but the underlying molecular mechanisms remain unclear. Nerve injury also causes enduring changes in the expression of pro- and anti-nociceptive genes,8,9 in addition to abnormal hyperactivity of primary sensory nerves.10 However, the molecular mechanisms involved in the sustained alterations in gene transcription and their roles in neuropathic pain are not fully elucidated.
Dimethylation of lysine 9 of histone H3 (H3K9me2) is a post-translational modification present in both euchromatin and heterochromatin typically at the promoters of silenced genes, which is catalyzed by an enzymatic complex comprising euchromatic histone methyltransferases G9a and G9a-like protein (Glp).11,12 Biologically, G9a/Glp is a master regulator of lineage-specific gene expression in the brain, which can repress gene expression and a crucial repressive enzymatic complex for governing transcriptional output during development and neuronal cell differentiation.13 Other published reports showed that downregulation of the activity of G9a by repeated cocaine administration mediates cocaine’s ability to regulate the dendritic spine density of neurons and plasticity and also result in a switch of neuronal subtypes, from dopamine (DA) receptor D2 expressing (Drd2) neurons to Drd1 neurons,14,15 demonstrating a critical role of G9a/Glp in cocaine-induced central plasticity. Meanwhile, as a DA reuptake inhibitor increasing neuronal activity,16 cocaine’s role in the nerve system is relied on its function on DA system by causing symptoms of increased DA-elevated energy and arousal and an experience of reward and pleasure.17,18 Therefore, we hypothesized that the reduced inhibition of dopaminergic transmission was from the downregulation of tyrosine hydroxylase (tyrosine hydroxylase, the rate-limiting enzyme in DA synthesis) expression by G9a/Glp complex through methylating its gene Th in peripheral nerve injury-induced neuropathic pain.
Methods and materials
Subjects and peri-surgical care
After approval by the Institutional Committee of Animal Care and Use, we used male C57BL/6 mice weighing 20–25 g, 7–9 weeks of age, for all behavioral tests in accordance to the guidance of the Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. The peri-surgical treatment of the animals was reported elsewhere previously.19 In brief, animals are housed in a cage on a reverse 12:12-h dark–light cycle at the housing temperature of 23 ± 1℃. After randomization, each animal was placed into a test box with three mirrored sides for habituation for 10 min, and then the test sessions took place. No food or water was available to the mice during the experiment. After the experiment, each animal was euthanized by administrating a lethal dose of pentobarbital.
Study protocol
All animals underwent plantation of intracerebroventriclar (i.c.v.) cannula seven days before nerve injury and were subjected to the mechanical behavioral tests at the day 1 before nerve injury, and the days of 0, 7, 14, 21, 28, 35, 42, and 49 after nerve injury. For the intrathecal treatment, BIX 01294 was dissolved in dimethyl sulfoxide (DMSO) and injected daily since the post-injury days 7 and 14 for consecutive three days, three weeks, or six weeks. During the interventional animals, the pain behaviors were measured at variable time points following different interventional protocols. All experimental drugs were injected daily intrathecally using a microsyringe in a volume of 5 µL over 30 s, and then 10 -µL saline was used to flush the catheter dead space. For the vehicle control animals, a total of 15 µL of DMSO only was given.
Plantation of intracerebroventricular cannula
A cannula was stereotaxically implanted to all mice to allow i.c.v. microinjections as reported previously.20 Briefly, under aseptic conditions, a hole was drilled in the skull that located at lateral 1.0 mm, posterior 0.5 mm to Bregma, and the tip of a 26-G stainless steel infusion cannula was placed 2.0 mm below the surface of the skull into the right ventricle. Then, the cannula was secured to the skull using dental cement and a stylus was inserted to maintain patency. The surgeries were performed under isoflurane anesthesia (4% induction; 1.5%–2% maintenance). A total of 30 mg/kg of Ibuprofen was available orally through drinking water for 24 h preoperatively and for at least 72 h postoperatively to alleviate possible postoperative pain. Sodium penicillin 10,000 IU (Shanghai AoBopharmtech, Shanghai, China) was given subcutaneously against infection. Finally, 0.4 mg of dexamethazone in 0.2 mL was given subcutaneously preoperatively to reduce brain swelling. The mice would be excluded if neurological deficits exhibited after the cannula plantation.
Spared nerve injury
The spared nerve injury (SNI)-induced model has been described elsewhere previously.21 In brief, animals were anesthetized with isoflurane (4% induction; 1.5%–2% maintenance), and the tibial and common peroneal branches of the sciatic nerve were ligated and then sectioned distally, but the sural nerve was left intact. Animals in the sham control underwent exposure of the sciatic nerve merely but were not ligated or dissected.
Mechanically evoked pain
Before and after surgery at different time points, mechanical pain thresholds were assessed from the withdrawal responses to the stimulation using the von Frey filaments. The test was carried out according to our previous study that has described the protocol.19 In brief, the surface of the hind paw plantar was probed with the filaments of varying forces were applied for a maximal of 10 s, and the stimulus threshold was calculated through evoking a quick withdrawal response. The stimulation started with 0.407 g and the increment was based on the withdrawal response to the current stimulation. If the withdrawal response appears, then the same filament strength was given 60 s later again; but if not, a higher strength was presented. If the mouse withdrew its hind paw in two consecutive trials with the same filament strength, then no further filaments were tested. The absolute response threshold was determined by fitting to a Gaussian Integral Psychometric Function via a Maximum Likelihood method.
Western blotting detection
The dorsal root ganglion (DRG), lumbar spinal cord L5–6 (L-SC), ventral tegmental area (VTA), nucleus accumbens (NAc), and prefrontal cortex (PFc) from sham and SNI mice were separated and minced into small pieces, and then suspended in lysis buffer (4% SDS, 0.004% bromophenol blue, 20% glycerol, Tris-HCl, 10% 2-mercaptoethanol, pH 6.8, containing 50 mM PMSF, 6 M urea, 50 mM Na3VO4, Sigma) for protein extraction. The Bradford method22 was used to determine the concentration of total protein. A rabbit polyclonal primary antibody (Biorbyt, Explore. Bioreagents.) at 1:1000 dilution was used to detect G9a, Glp, and TH. Following incubation and repeated washing, donkey anti-rabbit secondary antibody (IgG-HRP-1:10,000; Santa Cruz Biotechnology Inc) incubation was performed. Enhanced chemiluminescence was used to visualize the bands. Finally, protein levels were normalized in regard to the signal obtained with bactin monoclonal antibodies (Santa Cruz Biotechnology; 1:1,000 dilution) chosen as housekeeping protein, and then the protein expression values were expressed as mean of arbitrary unit ± SEM.
High-performance liquid chromatography
DA concentrations were analyzed using high-performance liquid chromatography (HPLC) equipment fitted with an electrochemical detector. The mobile phase comprised 0.15 mM NaH2PO4, 0.01 mM octyl sodium sulfate, 0.5 mM Ethylenediaminetetraacetic acid (EDTA), and 12.5% methanol and was delivered by the model 590 pump (Waters Associates, Milforal) at a rate of 1 mL/min into an Ultrasphere 3 mm ODS column. The electrochemical detector of HPLC was an ESA Coulochem mod. 5100A with a dual electrode analytical cell (mod. 5011). The mean dialysate concentration of six samples for each mouse in the present study was expressed as fmol in 10 mL of perfusate.
Bisulfite sequencing
Methylation data obtained from DRG, L-SC, VTA, NAc, and PFc of Sham and spinal nerve ligation (SNL) mice to investigate the methylation of CpG islands of the Th gene near the transcriptional start site. Genomic DNAs obtained from pools of above tissues were treated with bisulfite using an Epitek bisulfite kit. This process changes the unmethylated cytosine to uracil with the methylated cytosine unchanged. The region of interest close to the transcriptional start site of gene Th was amplified by polymerase chain reaction by using specific primers. The amplified DNA was cloned into 4-TOPO vector, and then the DNA was sequenced. We analyzed every sequence for the presence of methylated cytosines using the QUMA web-based program corresponding to a different allele Th CpG islands methylation.
Statistical analyses
Data are expressed as the means ± SEMs, and analyzed using GraphPad Prism version 5.0 (GraphPad Software Inc., San Diego, CA) or PASW Statistics v18.0 (IBM Co., Armonk, NY). Two-way analysis of variance was used to analyze nociceptive data and multiple group results with where treatment and nociceptive status or other multiple factors were regarded as independent variables, and all factors were between-subjects factors. For multiple comparisons among different points in time, the analysis of variance tests were always followed by the Bonferroni post hoc tests. Student’s t test was used to analyze the intergroup difference when there were two testing groups. Paired t test was used to analyze the expressions of proteins detected with Western Blotting. All P values reported are two sided and difference is considered significant at P < 0.05.
Results
G9a/Glp expression after SNI
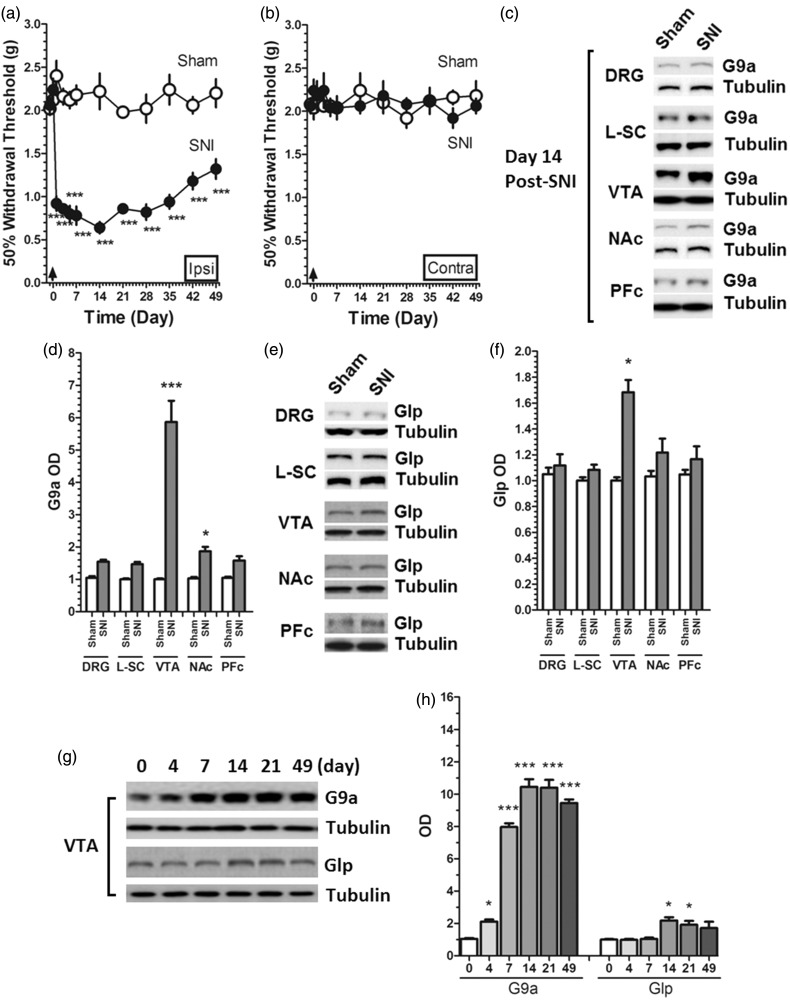
Mechanically evoked pain behaviors in SNI mice displayed statistical difference between the ipsilateral and the contralateral paws. The time course of mechanical withdrawal threshold showed that the nerve injury induced a time-dependent reduction of mechanical threshold and peaked at post-injury day 14 and then started recovery in the ipsilateral hind paw, but no significant change was observed in the contralateral limb (Figure 1(a) and (b)). To verify the nerve area involved in neuropathic pain, we measured the expression of G9a/Glp at post-injury day 14 at different nerve areas and found that G9a was upregulated in mice VTA but not other nerve areas at post-injury day 14 (Figure 1(c) and (d)). Based on this observation, we further measured G9a/Glp content in mice VTA after SNI and found that the expression of G9a but not Glp were upregulated in VTA at post-injury day 4 till day 49 (the last day of the behavioral test) (Figure 1(e) and (f)). This suggested that the expression of G9a changed time-dependently in VTA after SNI.
Figure 1.
SNI-induced changes in pain threshold and G9a/Glp expression. (a, b) SNI-induced marked decrease of mechanical withdrawal threshold in the ipsilateral hind paw, but no significant change was observed in the contralateral limb (***P < 0.001 vs. corresponding time points in Sham); (c, d) G9a was upregulated in mice cerebrospinal regions at post-injury day 14, and in both VTA and NAc, the expression of G9a showed statistically significant changes (*P < 0.05 and ***P < 0.001 vs. Sham); but in DRG (P = 0.052 vs. Sham), L-SC (P = 0.06 vs. Sham), and PFc (P = 0.051 vs. Sham), the expression of G9a was upregulated, but did not show statistical significance; (e, f) Glp was upregulated only in VTA at post-injury day 14 (*P < 0.05 vs. Sham), but other regions did not show significant change. (g, h) The expression of G9a showed a time-dependent change in VTA at post-injury days (day 0 to day 49), but Glp only showed changes at days 14 and 21 after SNI (*P < 0.05 and ***P < 0.001 vs. day 0). Data were presented as means ± SEMs. The sample number of all behavioral and protein expression were 15 (n = 15).
Th CpG islands methylation at different cerebrospinal areas after SNI
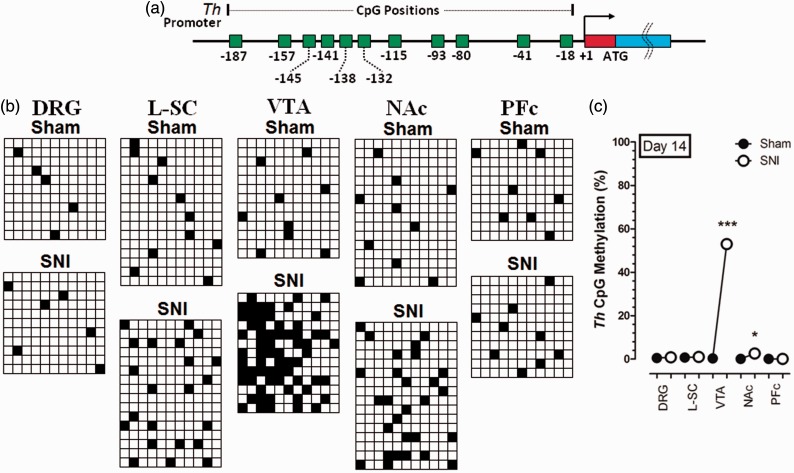
After measuring the expression of G9a/Glp in the SNI-injured animals, we next detected the methylation of Th CpG islands using bisulfite sequencing at different cerebrospinal areas on day 14 after SNI. The Th CpG islands methylation is increased markedly at post-injury day 14 in VTA, but no significant change was observed in other areas (Figure 2), indicating that VTA is the major area critically involved in the regulation of the methylation of Th gene.
Figure 2.
Changes of Th CpG methylation after SNI. (a) Eleven CpG islands of Th gene promoter and corresponding locations. (b, c) The Th CpG islands methylation is increased markedly at post-injury day 14 in the VTA, but no significant change was observed in DRG, L-SC, NAc, and PFc. Each row denotes one sample, and each column represents one CpG island. *P < 0.05 and ***P < 0.001 versus Sham. Data were presented as means ± SEMs; n = 11–16.
Th CpG islands methylation, TH expression, and DA level in VTA after SNI
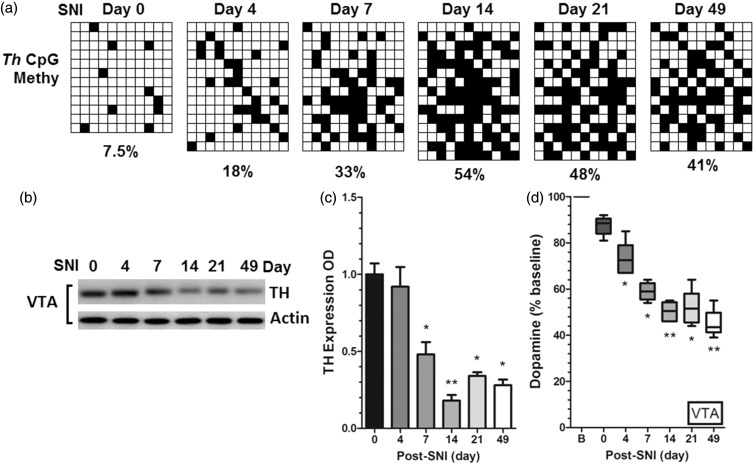
Based on previous findings of G9a/Glp expression and Th methylation in SNI mice, we next detected the alterations of Th CpG islands methylation, TH expression, and DA level in VTA at post-injury day 4 till day 49. Interestingly, the Th CpG islands methylation is also increased in a time-dependent manner (Figure 3(a)), but the TH expression was downregulated (Figure 3(b) and (c)) and the DA level was decreased correspondingly (Figure 3(d)), suggesting that dopaminergic transmission may be involved in the peripheral nerve injury-induced hypersensitivity. However, further investigations are needed to clarify and verify the role of G9a/Glp in this process.
Figure 3.
Time-dependent changes in dopaminergic pathway in VTA after SNI. (a) Th CpG islands methylation in VTA is increased after SNI. Each row denotes one sample, and each column represents one CpG island (n1 = 12–14); (b, c) TH expression in VTA was downregulated at post-injury day 7 till day 49 (*P < 0.05 and **P < 0.01versus post-SNI day 0); (d) DA level in VTA was downregulated at post-injury day 4 till day 49 (*P < 0.05 and **P < 0.01 versus post-SNI day 0). Data were presented as means ± SEMs; n2 = 15.
Effect of G9a/Glp inhibition on pain, Th methylation, and dopamine level in VTA
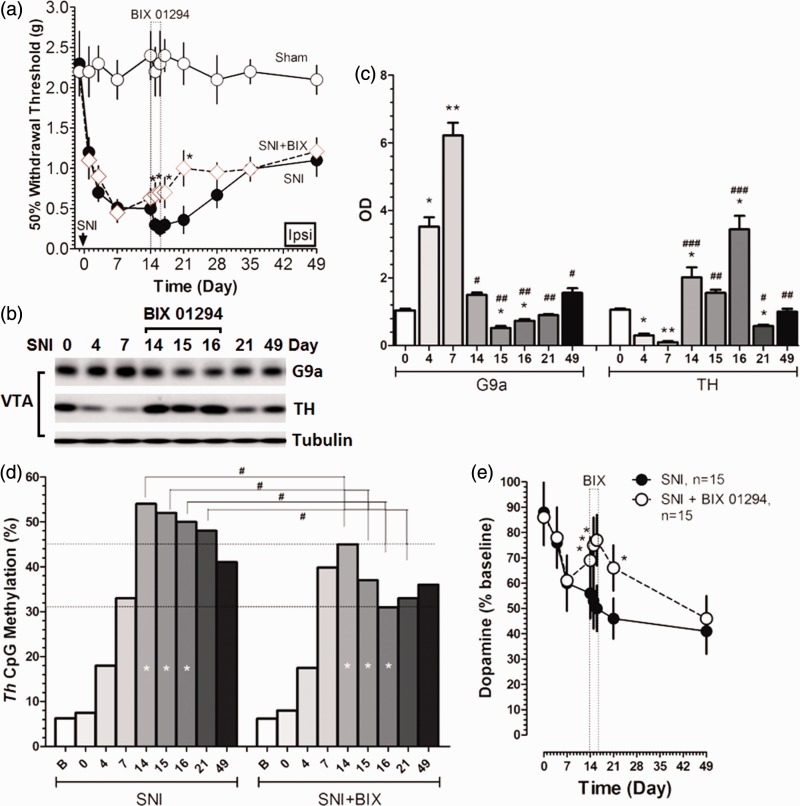
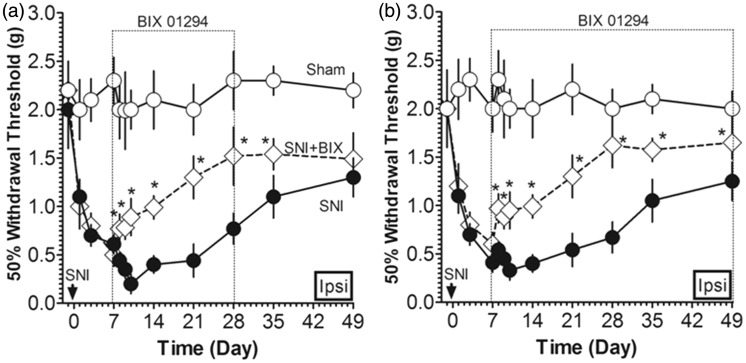
For further confirmation of G9a/Glp’s role in the reduced inhibition of dopaminergic transmission in peripheral nerve injury-induced neuropathic pain, we determined whether the inhibition of G9a/Glp affects von Frey-evoked pain, G9a and TH expression, CpG methylation, and DA level. We applied G9a/Glp inhibitor BIX 01294 i.c.v. to the mice after SNI and found that after the injection of BIX 01294 since the post-injury day 14 for consecutive three days, the expression of G9a was significantly downregulated (Figure 4(b) and (c)), but the expression of TH (Figure 4(b) and (c)) was upregulated with a significant decrease in Th methylation (Figure 4(d)) and the DA level was increased markedly (Figure 4(e)). Moreover, the G9a/Glp inhibitor administration elevated the pain threshold than the sole SNI animals (Figure 4(a)). These results indicate that G9a/Glp inhibition reversed the hyperexcitability in VTA, and the dopaminergic inhibition by G9a/Glp complex on TH expression may be involved in nerve injury-induced hypersensitivity.
Figure 4.
Effect of G9a/Glp inhibitor on pain, Th methylation, and dopamine in SNI animals. (a) The G9a/Glp inhibitor administration elevated the pain threshold than the sole SNI animals for consecutive three days (*P < 0.05 versus SNI); (b, c) G9a expression was downregulated, but the expression of TH was upregulated in VTA after G9a/Glp inhibitor i.c.v. injection (*P < 0.05 and **P < 0.01 versus SNI day 0; #P < 0.05 and ##P < 0.01 and ###P < 0.001 versus SNI day 7); (d) CpG methylation was decreased significantly in VTA after G9a/Glp inhibitor i.c.v. injection for consecutive three days (#P < 0.05 versus SNI); (e) DA level was increased in VTA after G9a/Glp inhibitor i.c.v. injection for consecutive three days (*P < 0.05 versus SNI). Data were presented as means ± SEMs; n = 15.
Effect of long-term inhibition of G9a/Glp on pain behavior
After measuring G9a/Glp inhibitor-induced changes since the post-injury day 14 for consecutive three days, we then applied G9a/Glp inhibitor BIX 01294 i.c.v. to the mice since the post-injury day 7 for consecutive three weeks and six weeks separately. As expected, the G9a/Glp inhibitor administration also considerably elevated the pain threshold than the sole SNI animals (Figure 5(a) and (b)). These data further confirmed that the increased level of G9a in VTA is an essential contributor to peripheral nerve injury-induced neuropathic pain, and inhibition of G9a/Glp may be a potential way of pain control.
Figure 5.
Long-term effect of G9a/Glp inhibition on pain threshold. The pain threshold significantly elevated in SNI animals after administration of G9a/Glp inhibitor i.c.v. for consecutive three weeks (a) and six weeks (b) (*P < 0.05 versus SNI). Data were presented as means ± SEMs; n = 15.
Discussion
In the present study, we tested the hypothesis that G9a/Glp complex played a role in the reduced inhibition of dopaminergic transmission in peripheral nerve injury-induced neuropathic pain via methylating the gene Th of TH and demonstrated that the expression of G9a but not Glp was upregulated in VTA after peripheral nerve injury. Following the changes of G9a, the methylation of the Th CpG islands is increased, but the TH expression was downregulated and the DA level was decreased. Further, after the i.c.v. injection of G9a/Glp inhibitor, the TH expression was upregulated with a significant decrease in Th methylation. Furthermore, the pain threshold after G9a/Glp inhibitor administration was elevated significantly. Our data suggested that the G9a/Glp complex is involved in the inhibition of DA in pain modulation through methylating the Th gene of TH.
An important knowledge gap in the research of neuropathic pain is whether epigenetic changes can initiate and then maintain abnormal gene expression in nerve injury-induced chronic pain. H3K9 methylation, a highly conserved histone post-translational modification, is commonly linked to transcriptional repression. G9a and Glp are the primary enzymes for H3K9me211,23 and exist predominantly as a G9a/Glp heteromeric complex that seems to be a functional H3K9 methyltransferase.12 Recently, Laumet et al.24 have reported that nerve injury increases the global expression level and activity of G9a and the enrichment of G9a-dependent H3K9me2, and then G9a inhibition restored the pain threshold to the baseline level gradually, providing functional evidence for the crucial role of G9a in neuropathic pain development. In addition, growing evidence shows that ventral striatum is critically involved in neuropathic pain25 and Brischoux et al.26 have also made great progress to unveil the changes of neural activity in response to acute noxious stimuli in brainstem areas, such as VTA. In this study, we measured the expression of G9a at post-injury day 14 at different nerve areas to verify the nerve area involved in neuropathic pain and found that G9a was upregulated at post-injury day 14 in mice VTA but not other nerve areas, so we tested G9a and Glp levels in VTA of mice and found that the expression of G9a but not Glp were upregulated in VTA at post-injury day 4 till day 49 after peripheral nerve injury, which is consist with the previous study.24
The descending inhibition involved in nociceptive information plays an important role in the control of excessive pain and the descending dopaminergic pathway is crucial during the process of antinociception. There are multiple evidences that pain perception can be controlled by dopaminergic pathways.27,28 Dopaminergic terminals have also been shown to modulate nociceptive transmission and to enhance antinociception.5,29 However, our study majorly concentrated on the supraspinal mesocorticolimbic dopaminergic pathway, rather than the spinal descending dopaminergic pathway. Our study revealed that the Th CpG islands methylation is increased, but the TH expression was downregulated and the DA level was decreased subsequently in VTA after SNI, suggesting that dopaminergic transmission may be involved in the peripheral nerve injury-induced neuropathic pain. However, further investigations will be required to clarify the role of dopaminergic receptors.
Changes in gene expression induced by nerve injury probably involve multiple epigenetic regulators, so, we here explored potential interaction between G9a/Glp in mediating pain sensitization and TH expression. Interestingly, study has shown that G9a inhibition restored the expression of many upregulated and downregulated genes that are involved in nociceptive signaling.26 In our study, after the injection of inhibitors of G9a/Glp, the expression of G9a was downregulated, but the TH expression was upregulated with a significant reduction in Th methylation and increase in DA level. Moreover, G9a/Glp inhibitor administration elevated the pain threshold than the sole SNI animals using i.c.v. injection for consecutive three days, three weeks, and six weeks. These data indicated that the G9a/Glp complex plays an inhibiting role in quenching the inhibition of DA in pain modulation through methylating the Th gene, and this disinhibition effect on dapaminergic transmission can produce effective alleviation of pain by G9a/Glp inhibitor suggesting that dopaminergic inhibition by G9a/Glp complex on TH may be involved in nerve injury-induced hypersensitivity. As demonstrated in the study by Dr Zhang et al.30 that the methyl CpG-binding protein 2 directly inhibiting G9a as a common mechanism in the amygdala for the regulation of pain and opioid reward in combination with our study suggesting that maybe some other pathways like dopamine-opioid pathway exist as DA’s joint players in determining the final behavior and the underlying mechanisms need further study.
Dr Laumet et al.24 have recently reported that G9a inhibition normalized genome-wide gene expression in the injured DRG and also significantly attenuated pain hypersensitivity in SNL rats and the underlying mechanism is associated with epigenetic silencing of K+ channel genes by G9a. Although this study also partially supports the concept that G9a/Glp is a crucial modulator of pain, the detailed findings were not consistent with ours. In contrast to Dr Laumet’s study, our study showed a little bit increase in the G9a expression in DRG, whereas did not detect significant change statistically (P = 0.052). For such a little change, we proposed that it may be associated with several aspects. First, the animal models were different. Both models, SNL and SNI, are widely used as representatives of neuropathic pain, but SNL has a propensity to induce more severe pain behavior with quicker onset and longer duration than that of the SNI, which displays relatively moderate changes correspondingly. Such difference in injury severity may be underlying the potential variations in protein expression at the same location. Second, when compared among different tissues (Figure 1(c) and (d)), the expression of G9a in VTA is much higher than the others, which guided us to look into the actual role of VTA G9a in SNI pain model, i.e., the supraspinal mechanisms of neuropathic pain. Of course, this research tendency does not mean other detected tissues in our study were not important for being investigated at length, but only not strong enough to be studied in the present research. Third, Dr. Laumet’s study also used genetically modified C57/BL6 mice (DRG G9a knockout) which undergone SNI, and showed substantial alleviation of pain indicating the contributing role of DRG G9a to SNI-induced pain, which reminds us that it is critically necessary to take further in-depth observations of G9a/Glp-associated contribution of potential genes’ epigenetic alterations to peripheral nerve injury-induced nociceptive hypersensitivity.
Before concluding, some limitations of the study should be acknowledged. DNA methylation, one of the major compositions of epigenetic modification, can undergo both direct and indirect modulations by methyltransferase.31,32 Direct modification indicates that the methyl group can be transferred to corresponding DNA CpG islands directly, whereas the indirect modification means that the methylated DNA CpG islands were secondarily resulted from the histone H3 lysine 9 dimethylation (H3K9me2), which was primarily methylated by methyltransferase like G9a/Glp complex. In our study, a correlation was observed between VTA G9a expression and Th methylation in SNI mice, but we did not identify whether this high methylation of Th CpG islands is directly from G9a/Glp or indirectly from G9a/Glp’s effect on H3K9me2 first. Furthermore, dopaminergic transmission is an essential part of central nervous system functional regulation. Currently, a total of eight supraspinal dopaminergic pathways have been identified33,34 and one spinal descending dopaminergic pathway.35 Although all these pathways are found being involved in the regulation of different types of pain to different extent, we in this study merely focused on the mesocorticolimbic pathway. As, thus, our findings in this study do not mean they are in common also to other dopaminergic pathways, especially for the spinal descending dopaminergic pathway, and should be cautious in explaining the role of G9a/Glp-associated dopaminergic transmission in peripheral nerve injury-induced pain. Last, in our study, we also found changes in Glp expression during days 14 to 21 after SNI, whereas we did not use Glp inhibitors to observe its role in this pain condition. So, whether Glp plays a role as G9a does in this SNI-induced hypersensitivity needs to be verified by further work.
Conclusions
In conclusion, our results for the first time suggest that methytransferase G9a/Glp complex partially controls dopaminergic transmission by methylating Th in peripheral nerve injury-induced neuropathic pain. The present findings in combination with other reports,24,30 advance our understanding of the G9a/Glp-associated epigenetic plasticity involved in the development of neuropathic pain. Moreover, these data provide clue that G9a/Glp may represent a promising target for preventing and treating peripheral nerve injury-induced neuropathic pain.
Author Contributions
Nan Wang, Xiaofeng Shen, Senzhu Bao, and Shan-Wu Feng contributed equally to this work.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Natural Scientific Foundation of China (NSFC, 81271242, 81371248, 81560200), Nanjing Municipal Medical Development Grant (QYK11139), and Nanjing Medical University Medical Science Development Grant (2014NMUZD043, 2014NJMU092).
References
- 1.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest 2010; 120: 3779–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turk DC, Wilson HD, Cahana A. Treatment of chronic noncancer pain. Lancet 2011; 377: 2226–2235. [DOI] [PubMed] [Google Scholar]
- 3.Millan MJ. Descending control of pain. Prog Neurobiol 2002; 66: 355–474. [DOI] [PubMed] [Google Scholar]
- 4.Tracey I, Dickenson A. SnapShot: pain perception. Cell 2012; 148: e1308–e1312. [DOI] [PubMed] [Google Scholar]
- 5.Taniguch W, Nakatsuka T, Miyazaki N, et al. In vivo patch-clamp analysis of dopaminergic antinociceptive actions on substantia gelatinosa neurons in the spinal cord. Pain 2011; 152: 95–105. [DOI] [PubMed] [Google Scholar]
- 6.Lapirot O, Melin C, Modolo A, et al. Tonic and phasic descending dopaminergic controls of nociceptive transmission in the medullary dorsal horn. Pain 2011; 152: 1821–1831. [DOI] [PubMed] [Google Scholar]
- 7.Cobacho N, de la Calle JL, Paíno CL. Dopaminergic modulation of neuropathic pain: analgesia in rats by a D2-type receptor agonist. Brain Res Bull 2014; 106: 62–71. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Sun H, Della Penna K, et al. Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience 2002; 114: 529–546. [DOI] [PubMed] [Google Scholar]
- 9.Xiao HS, Huang QH, Zhang FX, et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci USA 2002; 99: 8360–8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amir R, Michaelis M, Devor M. Burst discharge in primary sensory neurons: triggered by subthreshold oscillations, maintained by depolarizing afterpotentials. J Neurosci 2002; 22: 1187–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tachibana M, Sugimoto K, Nozaki M, et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev 2002; 16: 1779–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tachibana M, Ueda J, Fukuda M, et al. Histone methyltransferases G9a and GLP form heteromeric complexes and are both crucial for methylation of euchromatin at H3-K9. Genes Dev 2005; 19: 815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaefer A, Sampath SC, Intrator A, et al. Control of cognition and adaptive behavior by the GLP/G9a epigenetic suppressor complex. Neuron 2009; 64: 678–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maze I, Covington HE, 3rd, Dietz DM, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science 2010; 327: 213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maze I, Chaudhury D, Dietz DM, et al. G9a influences neuronal subtype specification in striatum. Nat Neurosci 2014; 17: 533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothman RB. High affinity dopamine reuptake inhibitors as potential cocaine antagonists: a strategy for drug development. Life Sci 1990; 46: PL17–PL21. [DOI] [PubMed] [Google Scholar]
- 17.Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci 1991; 14: 299–302. [DOI] [PubMed] [Google Scholar]
- 18.Ikemoto S, Wise RA. Rewarding effects of the cholinergic agents carbachol and neostigmine in the posterior ventral tegmental area. J Neurosci 2002; 22: 9895–9904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang F, Xu S, Shen X, et al. Spinal macrophage migration inhibitory factor is a major contributor to rodent neuropathic pain-like hypersensitivity. Anesthesiology 2011; 114: 643–659. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Wellman LL, Tang X, et al. Effects of corticotropin releasing factor (CRF) on sleep and body temperature following controllable footshock stress in mice. Physiol Behav 2011; 104: 886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 2000; 87: 149–158. [DOI] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 23.Rice JC, Briggs SD, Ueberheide B, et al. Histone methyltransferases direct different degrees of methylation to define distinct chromatin domains. Mol Cell 2003; 12: 1591–1598. [DOI] [PubMed] [Google Scholar]
- 24.Laumet G, Garriga J, Chen SR, et al. G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat Neurosci 2015; 18: 1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor AM, Murphy NP, Evans CJ, et al. Correlation between ventral striatal catecholamine content and nociceptive thresholds in neuropathic mice. J Pain 2014; 15: 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brischoux F, Chakraborty S, Brierley DI, et al. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci USA 2009; 106: 4894–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potvin S, Grignon S, Marchand S. Human evidence of a supra-spinal modulating role of dopamine on pain perception. Synapse 2009; 63: 390–402. [DOI] [PubMed] [Google Scholar]
- 28.Wood PB. Role of central dopamine in pain and analgesia. Expert Rev Neurother 2008; 8: 781–797. [DOI] [PubMed] [Google Scholar]
- 29.Munro G. Dopamine D1 and D2 receptor agonism enhances antinociception mediated by the serotonin and noradrenaline reuptake inhibitor duloxetine in the rat formalin test. Eur J Pharmacol 2007; 575: 66–74. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Tao W, Hou YY, et al. MeCP2 repression of G9a in regulation of pain and morphine reward. J Neurosci 2014; 34: 9076–9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Wijst MG, Venkiteswaran M, Chen H, et al. Local chromatin microenvironment determines DNMT activity: from DNA methyltransferase to DNA demethylase or DNA dehydroxymethylase. Epigenetics 2015; 10: 671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kvaratskhelia E, Tkemaladze T, Abzianidze E. Expression pattern of DNA-methyltransferases and its health implication (short review). Georgian Med News 2014; 228: 76–81. [PubMed] [Google Scholar]
- 33.Baik JH. Dopamine signaling in reward-related behaviors. Front Neural Circuits 2013; 7: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikkhah G. Restorative strategies for the dopaminergic nigrostriatal projection pathway. Acta Neurochir Suppl 2013; 117: 79–85. [DOI] [PubMed] [Google Scholar]
- 35.Sharples SA, Koblinger K, Humphreys JM, et al. Dopamine: a parallel pathway for the modulation of spinal locomotor networks. Front Neural Circuits 2014; 8: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]