Antibodies that inhibit binding of the merozoite invasion ligand EBA-175 are acquired by children exposed to Plasmodium falciparum and associated with protection from malaria. This identifies an important target and mechanism of antibody-mediated immunity to malaria, relevant to vaccine development.
Keywords: Plasmodium falciparum, malaria, EBA-175, binding inhibition, antibodies
Abstract
Background. The targets and mechanisms of human immunity to malaria are poorly understood, which poses a major barrier to malaria vaccine development. Antibodies play a key role in human immunity and may act by inhibiting receptor-binding functions of key merozoite invasion ligands. Antibodies to the major invasion ligand and vaccine candidate, erythrocyte-binding antigen 175 (EBA-175), have been linked with protection, but how these antibodies function has not been established.
Methods. We developed 2 new assays that quantify the ability of antibodies to inhibit binding of EBA-175 to its erythrocyte receptor, glycophorin A, using either native or recombinant EBA-175. Binding-inhibitory antibodies were evaluated in a longitudinal cohort study of Papua New Guinean children and related to risk of malaria, age, infection status, and markers of parasite exposure.
Results. Binding-inhibition assays (BIAs) were reproducible, and the 2 assays had a high level of agreement. Inhibitory antibodies were common among children, acquired in association with markers of increasing parasite exposure, and high in those children with active infection. Inhibitory antibodies correlated with total immunoglobulin G levels to the EBA-175 binding domain (region II). Importantly, binding-inhibitory antibodies were significantly associated with protection from symptomatic malaria when measured using either BIA.
Conclusions. Findings suggest that naturally acquired binding-inhibitory antibodies are an important functional mechanism that contributes to protection against malaria and further supports the potential of EBA-175 as a vaccine candidate. Identifying vaccines and approaches that induce potent binding-inhibitory antibodies may be a valuable strategy in the development of highly efficacious malaria vaccines.
(See the Editorial Commentary by John on pages 1253–4.)
Decreasing the burden of malaria and protecting susceptible populations remain global health priorities; an effective vaccine would greatly advance this goal [1]. The blood stage of the parasite life cycle causes symptomatic malaria, hence malaria vaccine development has had a strong focus on blood-stage vaccine candidates to prevent clinical illness and death [2]. Protective immunity develops after repeated natural exposure, and antibodies play a key role in immunity [3, 4]. This provides a strong rationale that the development of effective blood-stage vaccines is achievable and that defining protective targets and molecular mechanisms of acquired immunity is valuable [5]. Acquired human immunity appears to act by controlling and inhibiting replication of blood-stage parasites, preventing the development of high-density parasitemia and symptomatic illness [2, 3]. Merozoites are important targets of acquired antibodies, and these antibodies probably act, in part, by inhibiting erythrocyte invasion, opsonizing merozoites for phagocytosis, and fixing complement [2, 6–8]. However, the specific antigenic targets and protective effector mechanisms for an effective blood-stage vaccine remain elusive.
Currently, there is a lack of established functional assays that assess molecular interactions between antibodies and specific merozoite antigens, which has limited identification of targets of protective antibodies. Growth inhibition assays are commonly used but do not reliably predict protective immunity [9], are usually not antigen specific, and measure the cumulative effect of antibodies on the entire growth cycle without specifying whether antibodies inhibit merozoite invasion, schizont development, or rupture [5]. There is a strong need for antigen-specific functional assays that determine antibody activity at a molecular level.
We hypothesized that antibodies that inhibit the receptor-binding function of key merozoite invasion ligands would contribute to protective immunity by limiting parasite replication. Erythrocyte-binding antigen 175 (EBA-175) is a major invasion ligand that binds to the prominent erythrocyte molecule, glycophorin A. EBA-175 is a good model antigen to study binding-inhibitory antibodies as it is one of few antigens that has a defined role in invasion and has a known binding receptor [10, 11]. Human antibodies to EBA-175 have been associated with protective immunity [12, 13] and can inhibit parasite invasion [14, 15] as well as inhibit the binding of erythrocytes to COS-7 cells expressing EBA-175 [16]. EBA-175 is a leading vaccine candidate that was recently evaluated in phase 1 trials [17], which demonstrated vaccine safety and immunogenicity as well as the induction of in vitro growth inhibitory activity [17]. Understanding the role of binding-inhibitory antibodies to EBA-175 is necessary to understand its potential as a vaccine candidate. Evaluating binding-inhibitory antibodies for EBA-175 would allow similar approaches for its paralogues, EBA-140, EBA-181, and (erythrocyte-binding ligand - 1 [EBL-1]) [18], as well as other antigens that have receptor–ligand interactions. EBA-175 is a type 1 transmembrane protein with 6 extracellular regions [19]. Region II (RII) is the functional binding domain and comprises 2 cysteine-rich Duffy binding-like domains (F1 and F2) [10]. During invasion, EBA-175 is released from micronemes [20], and RII binds glycophorin A on the erythrocyte to stimulate rhoptry protein release and tight junction formation [21]. EBA-175 is then cleaved from the merozoite surface by PfROM4 and released into the intravascular space; cleavage is thought to be important for enabling other interactions and subsequent merozoite entry into the erythrocyte [22]. The function of region III-V (RIII-V) remains unknown, although antibodies to it can inhibit invasion [23].
Our aim was to study functional binding-inhibitory antibodies to EBA-175, quantify these responses in populations that acquire immunity, investigate the potential role of these antibodies in protective immunity, and evaluate assays that could be applied to EBA-175 vaccine trials. To achieve this, we developed 2 binding-inhibition assays (BIAs) and applied them in a longitudinal cohort of children in Papua New Guinea (PNG).
METHODS
Details of Human Samples
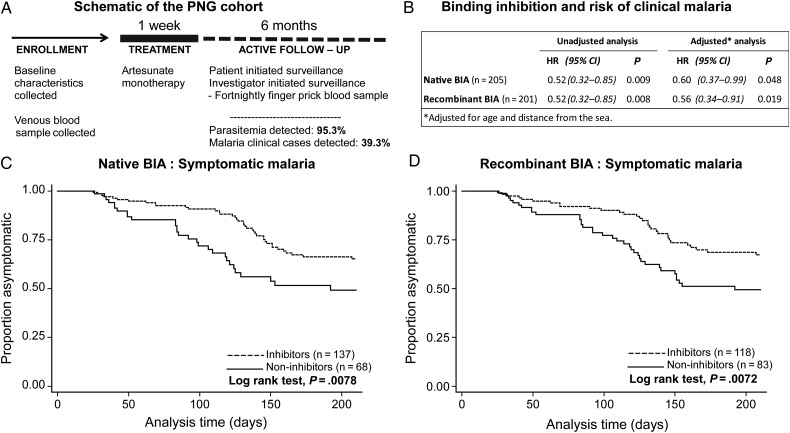
Plasma samples were obtained from a treatment reinfection cohort study involving 206 malaria-exposed children from PNG (aged 5–14 years), described previously [24]. At enrollment, demographic details were collected and venous blood samples were drawn. All children were treated with artesunate for 7 days to clear parasitemia. They were actively followed every 2 weeks for 6 months to determine episodes of asymptomatic reinfection and symptomatic malaria. During this time, 95.3% of participants became reinfected with Plasmodium falciparum and 39.3% developed clinical disease (fever >37°C and P. falciparum parasitemia >5000/µL).
Plasma from enrollment was tested using both BIAs for binding inhibition and a standard enzyme-linked immunosorbent assay (ELISA) protocol [25] for immunoglobulin G (IgG) to schizont lysate [12, 26] and EBA-175 RII (1 µg/mL in phosphate-buffered saline). Six months of prospective clinical follow-up was used to test for associations with protection. Samples from PNG adults were used as positive controls. Negative control samples were obtained from malaria-naive Australian blood donors.
Ethics Approval
Ethics approval was obtained from the PNG Institute of Medical Research Institute and the Alfred Hospital Human Research Ethics Committee. Informed consent was obtained from participants and their guardians.
Native Binding-Inhibition Assay
In brief, erythrocytes (0.4% hematocrit) were incubated with parasite culture supernatant at room temperature (RT) (30 minutes). EBA-175 binding was detected using polyclonal EBA-175 RIII-V rabbit antibody (Ab; 1/1000; 30 minutes RT), followed by anti-rabbit Alexa-488–conjugated Ab (1/1000; 30 minutes RT; Invitrogen). Mean fluorescence intensity was measured by flow cytometery (FACSCalibur, BD Biosciences). For binding inhibition, plasma (1/500) was incubated with the parasite supernatant prior to the binding step (30 minutes RT). Further details are provided in the Supplementary Methods.
Recombinant Binding-Inhibition Assay
In brief, native glycophorin A (8 µg/mL) was adsorbed onto F96 Maxisorp plates (overnight; 4°C; Nunc), then blocked (1% w/v BSA; 2 hours RT). Recombinant EBA-175 RII was incubated to allow binding (2 µg/mL; 2 hours RT), and this binding was detected using polyclonal EBA-175 RII rabbit sera (1/1000; 2 hours RT) [27], anti-rabbit horseradish peroxidase–conjugated Ab (1/500; 2 hours RT; Millipore), and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid liquid substrate (1 hour RT; Sigma). Optical density was measured at 405 nm. For binding inhibition, plasma (1/20) was incubated with EBA-175 RII prior to the binding step (30 minutes RT). Further details are provided in the Supplementary Methods.
Statistical Analyses
Individuals with binding-inhibitory antibodies were defined as those with binding responses lower than 3 standard deviations of the mean binding in the presence of malaria-naive controls (n = 12). BIA responses were not normally distributed; therefore, non-parametric statistical analyses were performed using StataSE 11 (StataCorp) and Prism 6 (GraphPad) software (details provided in the Supplementary Methods).
RESULTS
Development of Quantitative EBA-175 Binding-Inhibition Assays
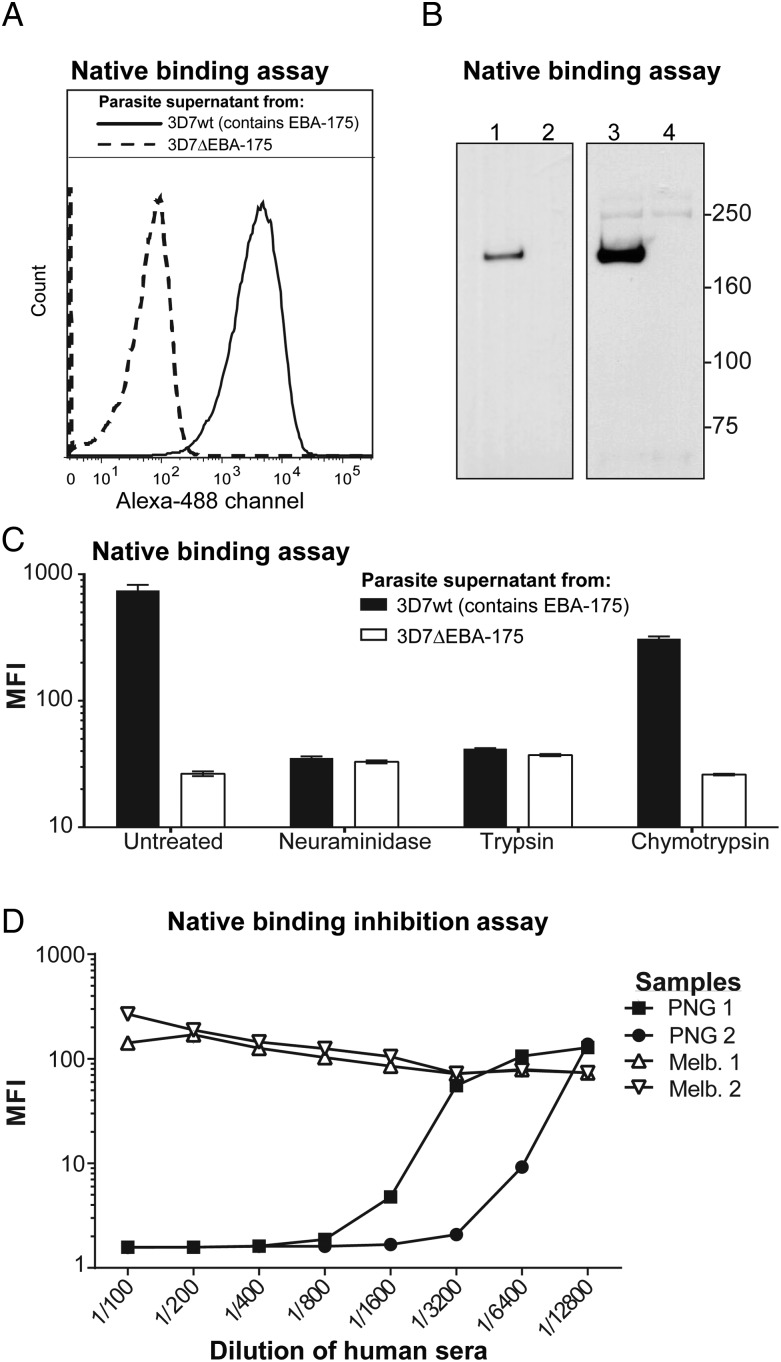
To investigate the acquisition of EBA-175 binding-inhibitory antibodies and their potential role in immunity, we developed a quantitative BIA using native EBA-175 protein and intact human erythrocytes (Supplementary Figure 1). Supernatants from in vitro parasite cultures were collected as the source of native EBA-175, and parasites lacking EBA-175 (3D7ΔEBA-175) were used as a control. This new assay used flow cytometry to demonstrate the binding of native EBA-175 protein to the erythrocyte surface (Figure 1A). Binding of EBA-175 was further confirmed by Western blotting of proteins eluted from the surface of erythrocytes (Figure 1B). Specificity of binding was demonstrated by absence of binding with supernatants from 3D7ΔEBA-175 parasites (Figure 1A and 1B) and by the expected pattern of binding inhibition following enzyme treatment of erythrocytes to cleave surface receptors; there was substantially reduced binding with neuraminidase or trypsin, but not chymotrypsin (Figure 1C) [10, 28]. A range of conditions was explored to optimize the assay, including concentration of parasite proteins, hematocrit (data not shown), and antibodies for detection of bound EBA-175 (Supplementary Figure 2). Human plasma antibodies were then tested for inhibition of EBA-175 binding across a range of concentrations. Samples from malaria-naive adults did not inhibit EBA-175 binding, whereas samples from malaria-exposed PNG adults effectively inhibited binding in a concentration-dependent manner (Figure 1D).
Figure 1.
Binding of native erythrocyte-binding antigen 175 (EBA-175) to erythrocytes and inhibition of binding by human antibodies. The degree and specificity of native EBA-175 binding to the surface of intact human erythrocytes was assessed by flow cytometry and Western blot. A, Native EBA-175 from the 3D7 wild-type parasite supernatant (solid line) and 3D7ΔEBA-175 negative controls (dashed line) were assessed with higher fluorescence (x axis), indicating higher EBA-175 binding. B, Western blots were used to confirm the presence or absence of native EBA-175 from parasite culture supernatants using 3D7 wild-type (lane 1) and 3D7ΔEBA-175 (lane 2), respectively. The ability of EBA-175 to bind to human erythrocytes was then assessed by coincubating the parasite supernatants with human erythrocytes, eluting off bound EBA-175, and detecting the presence of EBA-175 by Western blot using a rabbit anti–EBA-175 polyclonal antibody. Binding of EBA-175 was detected with a 175-kDa band when using 3D7wt supernatant (lane 3) but not with 3D7ΔEBA-175 supernatant (lane 4). A faint spectrin band can be seen at 250 kDa. C, The specificity of EBA-175 binding to erythrocytes was assessed following enzyme treatment of the erythrocytes with neuraminidase, trypsin, or chymotrypsin. These enzymes differentially cleave off sialic acids and glycophorin A. Culture supernatants from 3D7 wild-type (black bars) and 3D7ΔEBA-175 (white bars) parasites were used to detect the mean fluorescence intensity (MFI, y axis) of EBA-175 binding to human erythrocytes using the flow cytometry assay described earlier. Error bars show range for samples tested in duplicate. D, Human sera samples were used to assess the ability to inhibit the binding of EBA-175 to human erythrocytes in the flow cytometry assay described earlier, with binding-inhibitory samples yielding a low MFI (y axis). Representative samples included adults resident in malaria-endemic Papua New Guinea (PNG) (n = 2) and samples from malaria-naive blood donors resident in Melbourne, Australia (Melb.) (n = 2). The line graph shows a titration of sera (x axis) tested singly.
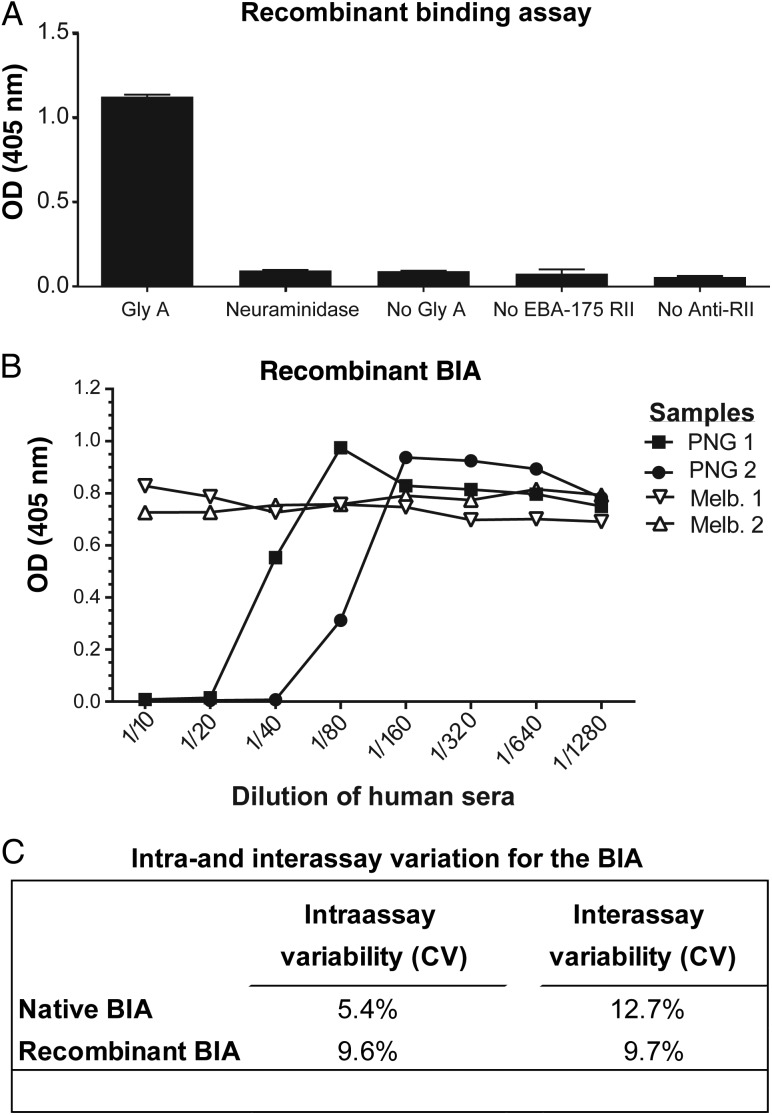
While the native EBA-175 BIA with erythrocytes best represents in vivo binding, a cell-free BIA would be ideal for application to large clinical studies and vaccine trials and would allow standardization of reagents. Therefore, a second assay was developed and optimized using recombinant EBA-175 RII and immobilized glycophorin A in a 96-well ELISA-based format (Supplementary Figure 1C). Conditions were explored to optimize the assay for application to clinical studies (Supplementary Figure 3A–E), and we confirmed binding of recombinant EBA-175 RII to erythrocytes (Supplementary Figure 3F). Specificity of binding was demonstrated by the absence of binding following enzyme treatment of glycophorin A with neuraminidase and other negative controls (Figure 2A). Following optimization of assay conditions (Supplementary Methods), human antibodies were tested for their ability to inhibit EBA-175 RII binding. As seen with the native BIA, samples from malaria-exposed PNG adults effectively inhibited binding in a concentration-dependent manner, whereas samples from malaria-naive adults did not (Figure 2B). Both BIAs showed low intraassay and interassay variability (Figure 2C) [29]. Individuals responded similarly in BIAs with native (full-length) and recombinant (RII only) EBA-175, suggesting that both assays could be used to evaluate EBA-175 binding inhibition. We confirmed that purified IgG from PNG donors, but not malaria-naive Melbourne residents, were able to inhibit EBA-175 binding (Supplementary Figure 4).
Figure 2.
Binding of recombinant erythrocyte-binding antigen 175 (EBA-175) to glycophorin A (Gly A) and inhibition of binding by human antibodies. The degree and specificity of recombinant EBA-175 (region II [RII]) binding to Gly A was assessed in an enzyme-linked immunosorbent assay–like approach that measures the degree of EBA-175 binding with an anti-EBA-175 rabbit polyclonal antibody with optical density (OD) measured at 405 nm. A, The specificity of recombinant EBA-175 binding to Gly A was assessed following neuraminidase treatment and compared with other negative controls (no Gly A, no EBA-175, no rabbit antibody for detection). Error bars show range for samples tested in duplicate. B, Human sera samples were used to assess the ability to inhibit the binding of EBA-175 to Gly A in the recombinant-binding assay described earlier with binding-inhibitory samples yielding a low OD (y axis). Representative samples included adults resident in malaria-endemic Papua New Guinea (PNG) (n = 2) and samples from malaria-naive blood donors resident in Melbourne, Australia (Melb; n = 2). The line graph shows a titration of sera (x axis) tested singly. C, Intraassay variability (within plate) and interassay variability (across plates) for both the native binding-inhibition assay (BIA) and recombinant BIA are shown as the average coefficient of variation (CV) for inhibitory and non-inhibitory samples.
PNG Children Develop Binding-Inhibitory Antibodies to EBA-175
The prevalence of EBA-175 binding-inhibitory antibodies was assessed in a longitudinal prospective cohort of 206 PNG children. Plasma at enrollment was tested in both BIAs and showed a wide range of responses (Supplementary Figure 5). The majority of individuals tested in native (67%) and recombinant (59%) BIAs had binding-inhibitory antibodies (Table 1). When responses of each assay were compared, there was a high level of agreement (Table 1; 86.0% agreement; Kappa =0.7017; P < .0001) and a significant correlation in levels of inhibitory activity (Table 1; Spearman rho = 0.7122; P < .0001).
Table 1.
Agreement Between Papua New Guinea Cohort Responses Tested With Native and Recombinant Binding-Inhibition Assays
| Native BIA |
Total (% of total) | ||
|---|---|---|---|
| Inhibitorsa | Non-inhibitorsa | ||
| Recombinant BIA | |||
| Inhibitors | 112 | 6 | 118 (59) |
| Non-inhibitors | 22 | 60 | 82 (41) |
| Total (% of total) | 134 (67) | 66 (33) | 200b (100) |
Cohen kappa test: agreement: 86.0%; kappa: 0.7017; P < .0001.
Abbreviation: BIA, binding-inhibition assay.
a Inhibitors were defined as binding responses lower than 3 standard deviations of the malaria-naive control.
b The total cohort included 206 children. All 206 samples were tested in the native BIA and 1 outlier was removed (native BIA analysis n = 205); however, this sample was included in the recombinant BIA analysis (1 non-inhibitor). Only 201 samples were tested in the recombinant BIA as 5 samples were depleted; however, these 5 samples were included in the native BIA analysis (3 inhibitors and 2 non-inhibitors in native BIA). These 6 samples are not presented in this table due to the nature of the analysis.
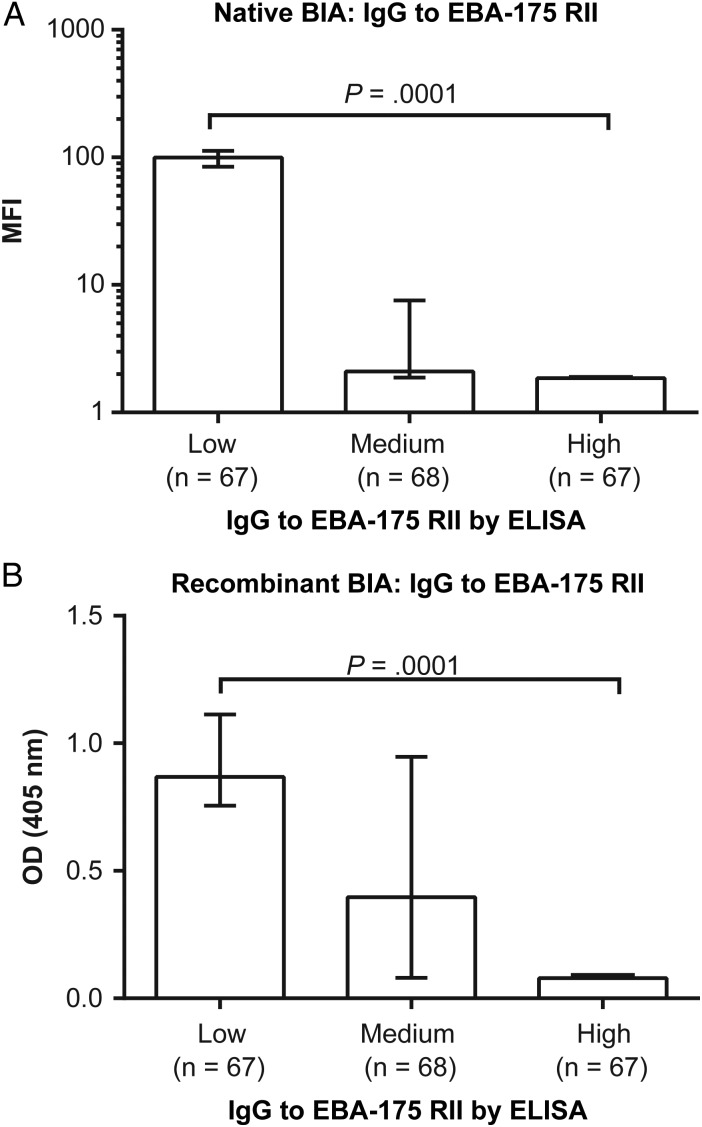
EBA-175 binding inhibition by antibodies was strongly related to EBA-175 IgG levels (measured to the RII binding region by ELISA); inhibition was highest among EBA-175 IgG high responders (defined as the upper tertile of responses) and lowest among the low-responder group (Figure 3). This is also reflected in the strong correlation between EBA-175 binding inhibition and IgG to EBA-175 RII (native BIA: Spearman rho = −0.853, P < .0001; recombinant BIA: Spearman rho = −0.704, P < .0001).
Figure 3.
Binding-inhibitory responses of Papua New Guinea (PNG) children and the relationship with immunoglobulin G (IgG) to erythrocyte-binding antigen 175 (EBA-175) region II (RII). Plasma samples from a cohort of PNG children were used to determine the relationship between EBA-175 binding inhibition and total IgG response to EBA-175 RII as determined by enzyme-linked immunosorbent assay (ELISA). The continuous values for IgG responses were used to divide the cohort into 3 equal categories reflecting low, medium, and high EBA-175 region II IgG responders (x axis). The relationship between these antibody levels and absolute binding inhibition was assessed using the (A) native binding inhibition assay (BIA) and (B) recombinant BIA. Greater degrees of binding inhibition were indicated by low mean fluorescent intensity (MFI) and low optical density (OD), respectively. Differences across all tertiles were first tested using a Kruskal–Wallis test (P = .0001), and further differences between groups (low, intermediate, or high) were tested using a Wilcoxon rank sum test (P < .0001 for all tests). Bar graphs indicate the median binding activity, and bars indicate the interquartile range.
Relationships Between EBA-175 Binding-Inhibitory Antibodies and Age, Infection, and Exposure
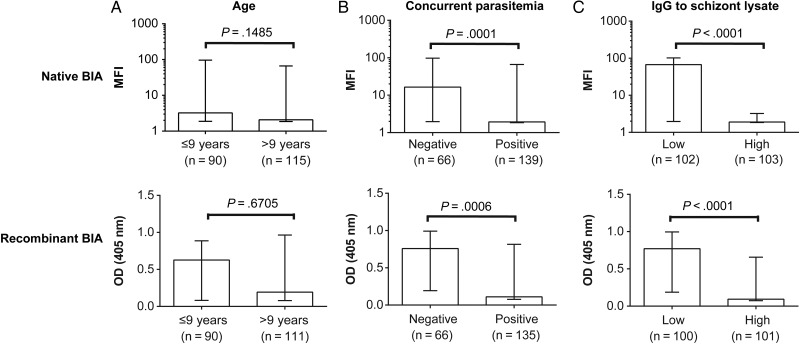
The magnitude of EBA-175 binding inhibition was assessed relative to the following markers of the acquisition of immunity: age, concurrent parasitemia, and antibodies to schizont protein extract (widely used as a broad marker of antibodies to blood-stage antigens, reflecting cumulative exposure and recent infection) [12, 30]. There was no significant association with age using a dichotomous variable of older vs younger children (Figure 4A) or with age as a continuous variable (native BIA: Spearman rho = −0.07, P = .3216; recombinant BIA: Spearman rho = 0.01, P = .9258). At enrollment, 67.5% of children were positive for P. falciparum infection by polymerase chain reaction (PCR) [24]. EBA-175 binding inhibition was significantly higher in the parasitemic group (Figure 4B), suggesting that inhibitory antibodies were boosted or induced by active infection. This trend was also observed when stratified by age (Supplementary Figure 6). EBA-175 binding inhibition was also significantly higher among children who were high responders to schizont protein extract (Figure 4C). Taken together, previous and active exposure appeared to contribute to the development of EBA-175 binding-inhibitory antibodies, but it is not closely linked with children's age.
Figure 4.
Binding-inhibitory responses of Papua New Guinea (PNG) children and relationship with age, concurrent parasitemia, and schizont protein extract immunoglobulin G (IgG) levels. Plasma samples from a cohort of PNG children were used to determine the relationship between erythrocyte-binding antigen 175 binding inhibition and (A) age, (B) concurrent parasitemia at the time of sample collection determined by polymerase chain reaction, and (C) IgG reactivity to schizont protein extract as a broad marker of antibodies to blood-stage antigens, reflecting cumulative exposure and recent infection. The relationship between these antibody levels and absolute binding inhibition was assessed using the native binding inhibition assay (BIA) and recombinant BIA; top and bottom panels, respectively. Greater degrees of binding inhibition were indicated by low mean fluorescent intensity (MFI) and low optical density (OD), respectively. Differences betweens groups was tested using a Wilcoxon rank sum test. Bar graphs indicate the median binding activity, and bars indicate the interquartile range. There was a significant association between increasing age group and schizont extract reactivity (P < .0001); this relationship remained significant when analysis was stratified by parasitemia status (parasitemic: P = .0026, n = 67; nonparasitemic: P = .0054, n = 139).
EBA-175 Binding-Inhibitory Antibodies Were Associated With Protection From Symptomatic Malaria
The design and longitudinal structure of the cohort study combined treatment to clear parasitemia at enrollment with 6 months of active follow-up to detect reinfection and clinical malaria cases (Figure 5A). This design enabled us to prospectively assess the relationship between EBA-175 binding-inhibitory antibodies and symptomatic malaria and to test the hypothesis that binding-inhibitory antibodies contribute to protective immunity or act as valuable biomarkers of such immunity. Children with EBA-175 binding-inhibitory antibodies had a significantly reduced risk of developing symptomatic malaria compared with children without EBA-175 binding-inhibitory antibodies (Figure 5B–D), and this association was found when results from the native and recombinant BIAs were used (native BIA: unadjusted hazard ratio [HR] = 0.52, 95% confidence interval [CI], [.32, .85], P = .009; recombinant BIA: unadjusted HR = 0.52, 95% CI, [.32, .85], P = .008). Previous analyses in this cohort identified age and residential location (distance from the sea) as potential confounders [12]. Adjustment for these factors had a minimal effect on the HRs, and results remained statistically significant (Figure 5B). Other studies have reported that parasitemia status at baseline can affect antibody levels and malaria risk [31]. While parasitemic individuals in this study had higher antibody levels at enrollment, parasitemia itself was not associated with malaria risk and is therefore not a significant confounder; adjustment of analyses for parasitemia at baseline did not substantially affect HRs (Supplementary Table 1). Individuals who were positive for inhibition in both assays had a significantly reduced risk of malaria compared with those who were negative in both assays (Supplementary Table 2).
Figure 5.
Binding-inhibitory responses of Papua New Guinea (PNG) children and risk of symptomatic malaria. A, A longitudinal cohort of 206 PNG children was treated to clear parasitemia after enrollment. Children were actively followed for 6 months to detect reinfection. B, Plasma from enrollment was tested in the native and recombinant binding-inhibition assays (BIAs). “Inhibitors” were defined as those with binding responses lower than 3 standard deviations of the malaria-naive control in each respective assay. Hazard ratios (HR) indicate the relationship between erythrocyte-binding antigen 175 binding inhibitors/non-inhibitors and their risk of symptomatic malaria over the 6-month active follow-up period. Kaplan–Meier curves show the relationship between groups of inhibitors and non-inhibitors from the (C) native BIA and (D) recombinant BIA and the time to first case of symptomatic malaria (fever >37°C and Plasmodium falciparum parasitemia >5000/µL). Differences between groups were tested using the log-rank test. Abbreviation: CI, confidence interval.
DISCUSSION
Merozoite antigens are important targets of acquired immunity and have significant potential as vaccine candidates. However, substantial challenges in identifying targets and mechanisms of protective antibody responses to guide vaccine development remain. There are currently no established antigen-specific functional assays that have been shown to correlate with protective immunity and that could be used for vaccine evaluation [5]. In this study, we developed assays to measure binding-inhibitory antibodies to the major merozoite invasion ligand and vaccine candidate, EBA-175. We show that binding-inhibitory antibodies to EBA-175 are acquired by children following exposure to malaria and appear to be boosted by active infection. Importantly, we demonstrate for the first time that binding-inhibitory antibodies are associated with protection from malaria in children.
To evaluate binding-inhibitory antibodies, we first developed an assay that would best represent physiological conditions using native EBA-175 and whole human erythrocytes. Quantification of binding by flow cytometry was a significant advantage over established Western blot approaches [28, 32, 33], especially for assessing large numbers of clinical samples (using <3 µL of plasma for each sample). The use of native EBA-175 made it possible to use the entire ectodomain of the antigen, increasing confidence in achieving correct conformational structure of the protein in vitro. This is important because until recently it had not been possible to recombinantly express full-length EBA-175, primarily because of its large size (approximately 1500 amino acids) [34]. A further benefit of using full-length native EBA-175 interacting with the dynamic bilipid membrane of the erythrocyte surface was to increase the ability to assess responses that inhibit more complex binding interactions, such as the predicted dimerization of EBA-175 with glycophorin A [35, 36]. Additionally, the recent finding that recombinant full-length EBA-175 binds glycophorin A with a 10-fold higher affinity than recombinant RII alone highlights the importance of developing 2 assays to compare inhibition between native (full length) and recombinant (RII) binding [34]. We developed our second assay using an ELISA-based approach with recombinant EBA-175 to facilitate standardization and quality control for application across future clinical studies and vaccine trials. The EBA-175 RII construct was recently used in phase 1 vaccine trials [17]; it should be possible to adapt this assay to use full-length recombinant protein that has recently been expressed [34].
Both BIAs performed well with a high level of precision and good agreement between the them. The correlation between the assay results suggests that most of the binding inhibitory activity is mediated by antibodies targeting RII epitopes. Results suggest that the recombinant BIA accurately predicts inhibition of native protein binding and would be sufficient for use in future clinical studies and vaccine trials. It is likely that the ability of human antibodies to inhibit EBA-175 is due to direct targeting of epitopes required for glycophorin A binding, epitopes that enable dimer formation, or epitopes outside of these regions yet close enough that steric hindrance of the antibody effectively blocks these interactions [37]. We also observed a strong correlation between total IgG EBA-175 RII responses (by standard ELISA) and binding-inhibitory activity. This suggests that there is an antibody concentration–dependent effect with binding inhibition, highlighting the importance of maintaining (or rapidly boosting) a memory response to obtain a high concentration of EBA-175 antibodies in order to mediate a binding-inhibitory effect to contribute to immunity. Further studies of these responses are needed to determine whether antibodies to EBA-175 measured by standard ELISA are a good surrogate measure of inhibitory activity.
This study is the first to demonstrate an association between binding-inhibitory antibodies to EBA-175 and protection from clinical malaria. The longitudinal study design was used to prospectively determine the relationship between antibodies and subsequent clinical illness [24]. Significant associations with protection remained after adjusting for potential confounders. It seems likely that binding-inhibitory antibodies act to limit P. falciparum invasion of erythrocytes and blood-stage replication, thereby preventing clinical illness. The functional activity of the antibodies and their prospective association with protection provide strong evidence for a role for antibodies to EBA-175 in protective immunity, as previously proposed [12]. We believe these findings are particularly significant because there are very few functional antibody responses that have been associated with protective immunity in children and because there is a lack of antigen-specific functional assays that are predictive of immunity. Although EBA-175 was the focus of this study, the approaches in this study also provide a basis to evaluate antibody-mediated binding inhibition for other merozoite antigens and advance the identification of key targets of protective human immunity. A smaller longitudinal study examined EBA-175 binding-inhibitory antibodies in adults (n = 81) in a high transmission area of Kenya where levels of immunity in adults are high [16]. Their assay assessed the ability of antibodies to inhibit binding of erythrocytes to COS-7 cells expressing EBA-175 RII. No association was found between symptomatic parasitemia and antibodies, but higher levels of binding-inhibitory antibodies were seen in adults who did not develop parasitemia compared with those who did. Our study results differ significantly in that we evaluated immunity in children, not adults. We also used a parasitemia density threshold for the diagnosis of malaria (as used by others [38, 39]) and PCR-based detection of parasitemia, neither of which was performed in the prior study.
In conclusion, our findings provide important evidence that EBA-175 binding inhibition by antibodies contributes to acquired human immunity and further supports EBA-175 as a vaccine candidate. Assays developed here have an obvious application in vaccine development of EBA-175 and possibly other antigens. Identifying vaccines and approaches that induce potent binding-inhibitory antibodies may be a valuable strategy in the development of highly efficacious malaria vaccines.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank all study participants and the Papua New Guinea Institute of Medical Research staff involved in the study; the Australian Red Cross Blood service for providing whole blood samples; Annie Mo (National Institutes of Health) for providing recombinant EBA-175 RII protein; and Jennifer Thompson, Julie Healer, and Alan Cowman for RIII-V antibodies (R1021) and 3D7ΔEBA-175 transgenic parasites. We also thank David Narum and Rosemary Ffrench for helpful discussions.
Financial support. This work was supported by the National Health and Medical Research Council of Australia (grant to J. G. B., Postgraduate Research Fellowship to J. S. R., and Infrastructure for Research Institutes Support Scheme Grant to Burnet Institute); the Australian Research Council (Future Fellowship to J. G. B.); the Victorian State Government Operational Infrastructure Support, University of Melbourne (Melbourne International Fee Remission Scholarship and Melbourne International Research Scholarship to V. I.); and Monash University (Australian Postgraduate Award to A. J. G. and Medicine, Nursing and Health Science International Honours Scholarship to V. I.).
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.WHO. Global Malaria Programme: World Malaria Report 2013. November 2013 ed. Geneva: World Health Organisation, 2013. [Google Scholar]
- 2.Richards JS, Beeson JG. The future for blood-stage vaccines against malaria. Immunol Cell Biol 2009; 87:377–90. [DOI] [PubMed] [Google Scholar]
- 3.Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol 2006; 28:51–60. [DOI] [PubMed] [Google Scholar]
- 4.Doolan DL, Dobano C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev 2009; 22:13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beeson JG, Fowkes FJI, Reiling L, Osier FH, Drew DR, Brown GV. Correlates of protection for Plasmodium falciparum malaria vaccine development: current knowledge and future research. In: Corradin G., Engers H., eds. Malaria vaccine development: over 40 years of trials and tribulations. UK: Future Medicine Ltd, 2014:80–104. [Google Scholar]
- 6.Osier FH, Feng G, Boyle MJ et al. . Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med 2014; 12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druilhe P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med 1995; 182:409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle Michelle J, Reiling L, Feng G et al. . Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity 2015; 42:580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan CJ, Hill AV, Ellis RD. Can growth inhibition assays (GIA) predict blood-stage malaria vaccine efficacy? Hum Vaccin Immunother 2012; 8:706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sim BK, Chitnis CE, Wasniowska K, Hadley TJ, Miller LH. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science 1994; 264:1941–4. [DOI] [PubMed] [Google Scholar]
- 11.Tham W-H, Healer J, Cowman AF. Erythrocyte and reticulocyte binding-like proteins of Plasmodium falciparum. Trends Parasitol 2012; 28:23–30. [DOI] [PubMed] [Google Scholar]
- 12.Richards JS, Stanisic DI, Fowkes FJ et al. . Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis 2010; 51:e50–60. [DOI] [PubMed] [Google Scholar]
- 13.McCarra MB, Ayodo G, Sumba PO et al. . Antibodies to Plasmodium falciparum erythrocyte-binding antigen-175 are associated with protection from clinical malaria. Pediatr Infect Dis J 2011; 30:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Persson KE, Fowkes FJ, McCallum FJ et al. . Erythrocyte-binding antigens of Plasmodium falciparum are targets of human inhibitory antibodies and function to evade naturally acquired immunity. J Immunol 2013; 191:785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badiane AS, Bei AK, Ahouidi AD et al. . Inhibitory humoral responses to the Plasmodium falciparum vaccine candidate EBA-175 are independent of erythrocyte invasion pathway. Clin Vaccine Immunol 2013; 20:1238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohas EA, Adams JH, Waitumbi JN et al. . Measurement of antibody levels against region II of the erythrocyte-binding antigen 175 of Plasmodium falciparum in an area of malaria holoendemicity in western Kenya. Infect Immun 2004; 72:735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Sahly HM, Patel SM, Atmar RL et al. . Safety and immunogenicity of a recombinant nonglycosylated erythrocyte binding antigen 175 region II malaria vaccine in healthy adults living in an area where malaria is not endemic. Clin Vaccine Immunol 2010; 17:1552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams JH, Blair PL, Kaneko O, Peterson DS. An expanding ebl family of Plasmodium falciparum. Trends Parasitol 2001; 17:297–9. [DOI] [PubMed] [Google Scholar]
- 19.Adams JH, Sim BK, Dolan SA, Fang X, Kaslow DC, Miller LH. A family of erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci U S A 1992; 89:7085–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sim BK, Toyoshima T, Haynes JD, Aikawa M. Localization of the 175-kilodalton erythrocyte binding antigen in micronemes of Plasmodium falciparum merozoites. Mol Biochem Parasitol 1992; 51:157–9. [DOI] [PubMed] [Google Scholar]
- 21.Aikawa M, Miller LH, Johnson J, Rabbege J. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J Cell Biol 1978; 77:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Donnell RA, Hackett F, Howell SA et al. . Intramembrane proteolysis mediates shedding of a key adhesin during erythrocyte invasion by the malaria parasite. J Cell Biol 2006; 174:1023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Healer J, Thompson JK, Riglar DT et al. . Vaccination with conserved regions of erythrocyte-binding antigens induces neutralizing antibodies against multiple strains of Plasmodium falciparum. PLoS One 2013; 8:e72504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michon P, Cole-Tobian JL, Dabod E et al. . The risk of malarial infections and disease in Papua New Guinean children. Am J Trop Med Hyg 2007; 76:997–1008. [PMC free article] [PubMed] [Google Scholar]
- 25.Richards JS, Arumugam TU, Reiling L et al. . Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J Immunol 2013; 191:795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanisic DI, Richards JS, McCallum FJ et al. . Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun 2009; 77:1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narum DL, Haynes JD, Fuhrmann S et al. . Antibodies against the Plasmodium falciparum receptor binding domain of EBA-175 block invasion pathways that do not involve sialic acids. Infect Immun 2000; 68:1964–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camus D, Hadley TJ. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 1985; 230:553–6. [DOI] [PubMed] [Google Scholar]
- 29.ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2(R1). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), 2005. [Google Scholar]
- 30.Ondigo BN, Hodges JS, Ireland KF et al. . Estimation of recent and long-term malaria transmission in a population by antibody testing to multiple Plasmodium falciparum antigens. J Infect Dis 2014; 210:1123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polley SD, Mwangi T, Kocken CH et al. . Human antibodies to recombinant protein constructs of Plasmodium falciparum apical membrane antigen 1 (AMA1) and their associations with protection from malaria. Vaccine 2004; 23:718–28. [DOI] [PubMed] [Google Scholar]
- 32.Liang H, Narum DL, Fuhrmann SR, Luu T, Sim BK. A recombinant baculovirus-expressed Plasmodium falciparum receptor-binding domain of erythrocyte binding protein EBA-175 biologically mimics native protein. Infect Immun 2000; 68:3564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang L, Gaur D, Mu J, Zhou H, Long CA, Miller LH. Evidence for erythrocyte-binding antigen 175 as a component of a ligand-blocking blood-stage malaria vaccine. Proc Natl Acad Sci U S A 2011; 108:7553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wanaguru M, Liu W, Hahn BH, Rayner JC, Wright GJ. RH5-basigin interaction plays a major role in the host tropism of Plasmodium falciparum. Proc Natl Acad Sci U S A 2013; 110:20735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salinas ND, Paing MM, Tolia NH. Critical glycosylated residues in exon three of erythrocyte glycophorin a engage Plasmodium falciparum EBA-175 and define receptor specificity. MBio 2014; 5:e01606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolia NH, Enemark EJ, Sim BK, Joshua-Tor L. Structural basis for the EBA-175 erythrocyte invasion pathway of the malaria parasite Plasmodium falciparum. Cell 2005; 122:183–93. [DOI] [PubMed] [Google Scholar]
- 37.Chen E, Paing MM, Salinas N, Sim BK, Tolia NH. Structural and functional basis for inhibition of erythrocyte invasion by antibodies that target Plasmodium falciparum EBA-175. PLoS Pathog 2013; 9:e1003390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mwangi TW, Ross A, Snow RW, Marsh K. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis 2005; 191:1932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bejon P, Berkley JA, Mwangi T et al. . Defining childhood severe falciparum malaria for intervention studies. PLoS Med 2007; 4:e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.