Abstract
Background:
Computed tomography (CT) guided biopsies have long been the standard technique to obtain tissue from the thoracic cavity and is traditionally performed by interventional radiologists. Ultrasound (US) guided biopsy of pleural-based lesions, performed by pulmonologists is gaining popularity and has the advantage of multi-planar imaging, real-time technique, and the absence of radiation exposure to patients. In this study, we aim to determine the diagnostic accuracy, the time to diagnosis after the initial consult placement, and the complications rates between the two different modalities.
Methods:
A retrospective study of electronic medical records was done of patients who underwent CT-guided biopsies and US-guided biopsies for pleural-based lesions between 2005 and 2014 and the data collected were analyzed for comparing the two groups.
Results:
A total of 158 patients underwent 162 procedures during the study period. 86 patients underwent 89 procedures in the US group, and 72 patients underwent 73 procedures in the CT group. The overall yield in the US group was 82/89 (92.1%) versus 67/73 (91.8%) in the CT group (P = 1.0). Average days to the procedure was 7.2 versus 17.5 (P = 0.00001) in the US and CT group, respectively. Complication rate was higher in CT group 17/73 (23.3%) versus 1/89 (1.1%) in the US group (P < 0.0001).
Conclusions:
For pleural-based lesions the diagnostic accuracy of US guided biopsy is similar to that of CT-guided biopsy, with a lower complication rate and a significantly reduced time to the procedure.
Keywords: Computed tomography-guided, pleural-based lesions, ultrasound guided
INTRODUCTION
With the introduction of lung cancer screening, pulmonary nodules will be encountered with increasing frequency, smaller sizes, and some of those will be located peripherally. The diagnosis of peripheral lung lesions is challenging as the yield of flexible bronchoscopy and sputum cytology is relatively low.[1,2,3] For pleural-based lung lesions, fluoroscopy computed tomography (CT) scans, and ultrasound (US) guided biopsy have been used to obtain adequate diagnostic tissue.[4,5,6] Electromagnetic navigational bronchoscopy and endobronchial radial US are increasingly being used to diagnose peripheral lung nodules, but there is little information about their utility in diagnosing pleural-based lesions. The US can visualize pleural-based thoracic masses, but remains underutilized as a diagnostic technique despite the fact that it has a high diagnostic yield, provides real-time guidance, can be performed at patients’ bedside and does not expose the patient to radiation.[5,6,7,8,9,10,11] Point-of-care US is now frequently used in clinical practice with portable, lightweight, affordable US machines with excellent imaging, readily available in many centers.[12] Lung sonography has become a valuable tool for pulmonologists, and many centers have US training as a part of their curriculum.[13,14]
The goal of this study was to conduct a retrospective review of US-guided transthoracic biopsies performed by pulmonologists versus CT-guided biopsies performed by interventional radiologists for pleural-based lung lesions. The diagnostic yield, days to procedure and complication rate were evaluated between the two groups. The local institutional review board approved the study.
METHODS
Study design and population
This is a single-center, retrospective, observational study of patients who underwent a biopsy of a pleural based lung lesion. Patients who had undergone CT or US guided biopsies of pleural-based lung lesions from 1997 to 2014 were included in the study. 89 US guided biopsies in 86 patients and 73 CT-guided biopsies in 72 patients were performed in patients with pleural-based lesions. The following data were collected: Demographics, the date of initial imaging showing the lesion, date the consult was placed to pulmonary or interventional radiology service, date of procedure, pathology report, size and location of the lesion, and procedural complications.
Procedure technique
Ultrasound guided biopsy
US-guided biopsies were performed using a Micro Maxx (Sonosite Inc., Bothell, WA, USA) unit equipped with a 1–5-MHz phased array transducer. The patients were placed in a comfortable position depending on the tumor location and patient preference, which included supine, lateral decubitus, or sitting upright with arms resting on a table. The lesion was located by scanning the intercostal spaces, and Doppler was used to certify the absence of blood vessels in the biopsy path. The biopsy site was disinfected with ChloraPrep (2% chlorhexidine, 70% isopropyl alcohol; Enturia Inc., Leawood, KS). Under local anesthesia and real-time guidance with US, fine needle aspiration biopsy (FNAB) samples were acquired [Figure 1]. An immediate post-procedure US was done to detect a pneumothorax. Patients were monitored in the recovery room for an hour where chest radiography was done.
Figure 1.
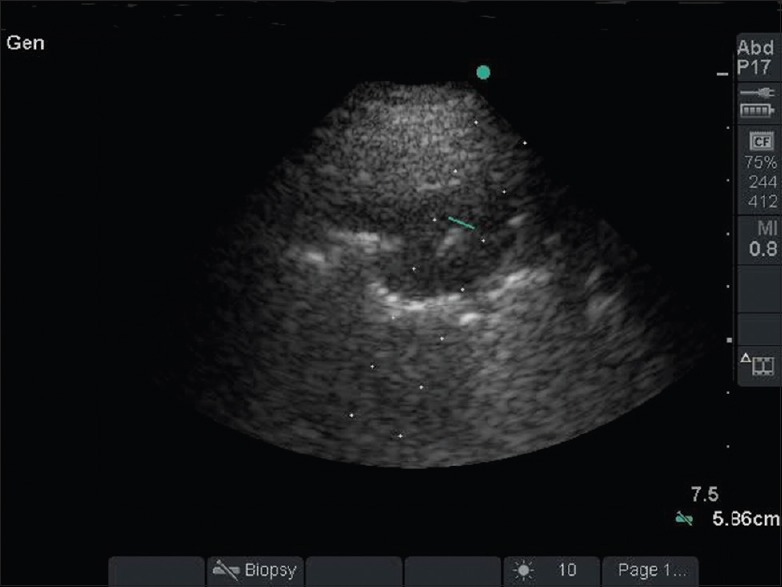
Ultrasound-guided biopsy of the left lower lobe pleural based lesion as seen on computed tomography scan
Computed tomography guided biopsy
CT guidance was performed by using a16-slice multi-detector CT scanner (Somatom Definitions AS, Siemens Medical Solutions, Malvern, PA). Patients were positioned on the CT table in a prone, supine, or lateral decubitus position, depending on the location of the lesion, to obtain the most direct route for biopsy. The skin entry site was marked by using the laser light from the CT gantry. The biopsy site was disinfected with ChloraPrep (2% chlorhexidine, 70% isopropyl alcohol; Enturia Inc., Leawood, KS, USA). Local anesthesia was infiltrated, and a19G Coaxial introducer needle (Gallini Medical Devices, Grand Rapids, MI, USA) was introduced, through which biopsy needles were advanced and once positioned, CT scan images were obtained to confirm location and samples were obtained [Figure 2]. Postbiopsy CT scan images are obtained to look for pneumothorax, and the patient is placed in a dependent position, with biopsy side down for 2 h.
Figure 2.
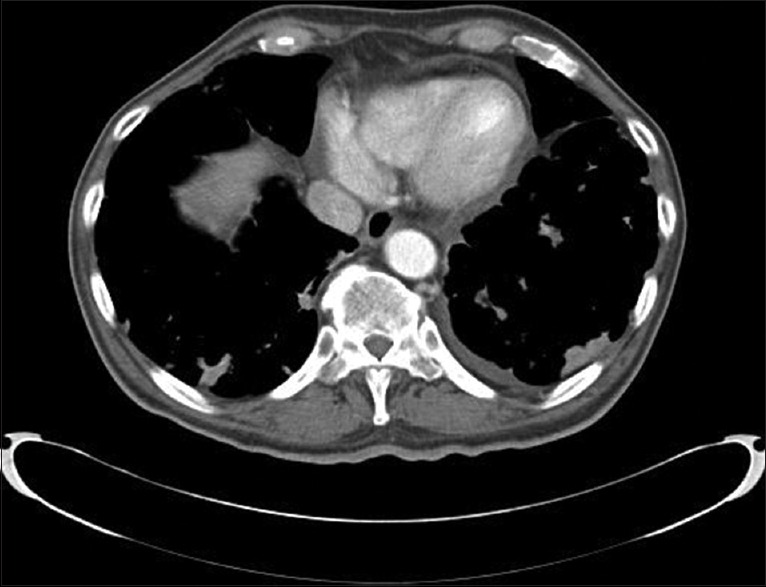
Bilateral parenchymal nodules with some of them pleural based
FNAB samples were obtained using 22G Franseen lung biopsy needle (COOK Medical, Bloomington, IN; length range 10–15 cm) and core biopsy was performed using a 20G Spring loaded biopsy needle (Gallini Medical Devices, Grand Rapids, MI; length range 10–15 cm) in both techniques.
Specimen processing
FNAB specimen was processed immediately by an onsite cytopathologist, and the core biopsy specimen was placed in a formaldehyde container [Figure 3].
Figure 3.
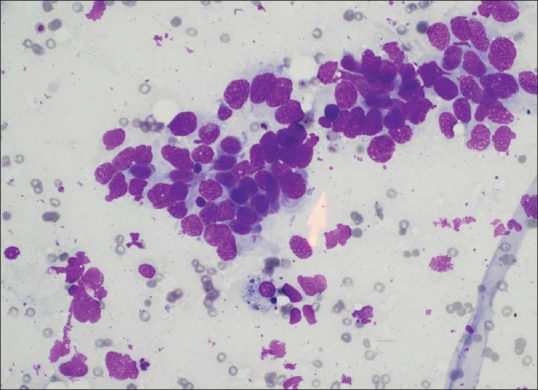
Nonsmall cell carcinoma diagnosed with ultrasound-guided fine needle aspiration biopsy
Data analysis
The maximum lesion size, age, and days to the procedure in the two groups were compared using the Mann–Whitney U-test. The diagnostic yield, complication rate, sex and race in the two groups were compared using the Fischer exact test. For a diagnosis of malignancy, the sensitivity, specificity, positive predictive value, and the negative predictive value were calculated for the two groups. P < 0.05 was considered statistically significant. All statistical computations were performed using GraphPad Instat version 3 statistical software (Graph Pad Software, Inc. La Jolla, CA, USA).
RESULTS
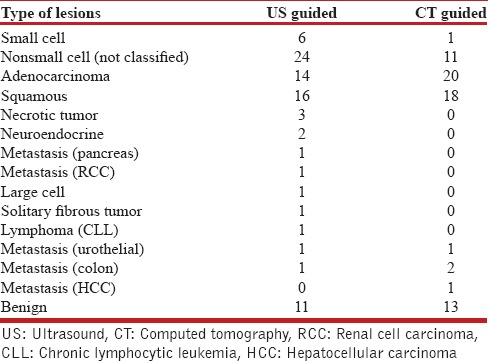
There were a total of 158 patients in the study [Table 1], 86 in the US-guided group and 72 in the CT-guided group. In the CT-guided group, 73 procedures were performed in 72 patients. There were 69 (95.8%) males and 3 (4.2%) females, with a mean age of 70.7 years (range 49–96). The mean size of the lesions was 4.9 cm (range 1.1–15 cm) and a median size of 4.3 cm (interquartile range [IQR], 3–6.4 cm). The diagnostic yield was 91.8% (67/73), with 54 (73.9%) malignant and 13 (17.8%) benign lesions [Table 2]. There were six (8.2%) false negatives with the final diagnosis found with a second CT-guided biopsy, a bone biopsy, and surgical resection in three cases respectively. Two patients were diagnosed with malignancy and received treatment at another facility and one patient died prior to establishing a diagnosis, but a review of his follow-up CT scan showed a significant increase in the size of the lesion, suggesting a high likelihood of malignancy. The average days to the procedure was 17.5 days (range 0–78 days) and the median days to the procedure was 15 days (IQR, 3–27 days). There was a total of 17 (23.2%) complications (13 pneumothorax, 2 hematoma at the biopsy site, one hemothorax, and one hemoptysis) [Table 3]. The pneumothorax resolved with needle aspiration in four patients, inhaled high flow oxygen and observation in eight while one patient required a chest tube. All patients with bleeding complications were observed for a day and then discharged without requiring intervention.
Table 1.
Study demographics
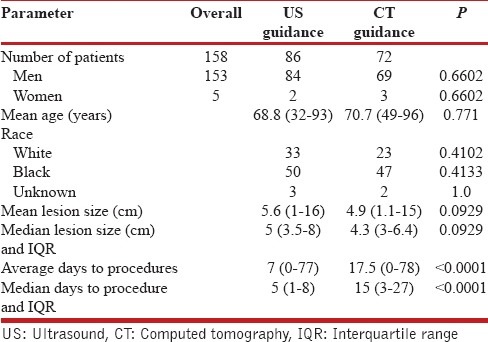
Table 2.
Diagnostic yield

Table 3.
Complications
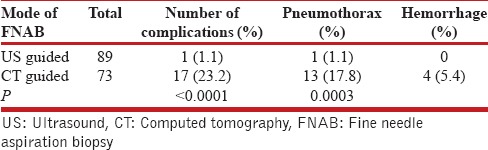
In the US group, there were a total of 86 patients who underwent 89 procedures. There were 84 (97.7%) males and two (2.4%) female, with a mean age of 68.8 years (range 32–93 years). The mean size of the lesion was 5.6 cm (range 1–16 cm) and the median size 5 cm (IQR, 3.5–8 cm). The diagnostic yield was 92.1% (82/89), with 71 (79.7%) malignant, and 11 (12.4%) benign. There was one (1.1%) false positive case, which was reported as a nonsmall cell carcinoma on US-guided biopsy, but on surgical resection it was found to be a lung abscess. There were a total of 6 (6.7%) false negative cases and the final diagnosis was established by surgical biopsy in three patients, repeat US-guided biopsy in two patients, and CT-guided biopsy in one patient. Details of diagnosis in each group are provided in Table 4. The mean days to the procedure were 7 (range 0–77 days) and the median days to procedure was 5 days (IQR, 1–8 days). One patient had a pneumothorax (1.1%) that did not require any intervention.
Table 4.
Type of lesions
Core biopsy and FNAB were performed in 52/73 (71.2%) procedures in the CT group compared to 20/89 (22.5%) in the US group (P < 0.0001). FNAB by itself was performed in 69/80 (77.5%) procedures in the US group compared to 21/73 (28.8%) in the CT group (P < 0.0001).
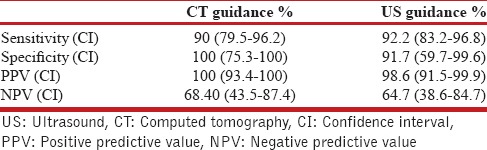
For a diagnosis of malignancy, the sensitivity, specificity, positive predictive value, and negative predictive values in the US and the CT group are provided in Table 5.
Table 5.
Diagnosis of malignancy
DISCUSSION
This is the first study to our knowledge to compare the US-guided biopsy with CT-guided biopsy for pleural-based lung lesions. In this study, we were able to show that US-guided biopsy has the same diagnostic yield as CT-guided biopsy, with fewer complications and a significantly reduced time to procedure. The reported diagnostic yield of US-guided biopsies for pleural-based lesions ranges from 64% to 97%[5,7,9,10,11,15] and that for CT-guided biopsy for peripheral lung lesions ranges from 80% to 95%[4,8,16] in the literature. In our study, the diagnostic yield of 91.8% and 92.1% in the CT-guided and US-guided group respectively was similar and comparable with those reported in the literature. Both, CT- and US-guided procedures had a high sensitivity, specificity and positive predictive value for diagnosing malignancy in our study. These results are also comparable to findings reported in other studies.[5,11,15]
Pneumothorax is the most common complication of CT-guided transthoracic biopsy with a reported frequency between 2.8% and 57%.[16,17] The rate of pneumothorax reported with US-guided biopsies is much lower in the published literature.[5,11,13,15,18] Our study too shows a significantly lower pneumothorax rate of 1.1% with US guidance compared to 17.8% with CT guidance (P = 0.0003). One could argue that the higher pneumothorax rate in studies of CT-guided biopsies of peripheral lung lesions include both pleural based and parenchymal lesions. There are no studies, besides ours, that compare the two techniques exclusively for pleural-based lung lesions. One hypothesis for the difference in pneumothorax rate in our study is that the real-time guidance offered by US-guided biopsies allows for an accurate puncture of the lesion as compared to CT-guided biopsies. Sconfienza et al.[11] in their study offer an additional explanation and suggest that a reduced procedural time of the US-guided biopsies compared to CT-guided biopsies along with real-time guidance in the US-guided group, helps prevent multiple punctures during sampling, thereby reducing complications. Although they did not have data on the number of punctures per procedure in their study, just as we do not have the same in our study. A second reason could be that the number of core biopsies in the CT-guided group was greater in our study. Core biopsies result in greater tissue trauma compared to FNAB and could have contributed to the higher pneumothorax rate. Sconfienza et al.[11] performed core biopsies in both groups and the complication rate was significantly higher in the CT-guided compared to the US-guided group (15.9% vs. 6.8%, P = 0.0272). Though in their study only 55 of the 170 patients had pleural-based lesions in the CT-guided group. In our study, patients in the CT-guided group who underwent a core biopsy and FNAB had a lower pneumothorax rate compared to those who only underwent FNAB (19.2%) compared to 33.3% respectively. Therefore, it is hard to explain the difference in pneumothorax rate based on biopsy technique. It is unclear if the smaller average lesion size (4.9 cm vs. 5.6 cm) in the CT-guided group in our study was a contributory factor for the higher pneumothorax rate in this group.
Studies report a shorter procedure time when US-guidance was used.[11,19] We did not evaluate the same in our study, as it was retrospective in nature and accurate procedure times were not recorded in the charts. Patient comfort and cooperation is increased when procedure time is reduced. Smaller lesions behind bony structures at times require a breath hold for them to be visualized with US and to be accurately punctured with either technique. Patients with dyspnea may not be able to hold their breath for too long, which can result in inadequate samples or accidental puncture of the lung.
We did evaluate the average days to procedure, and it was significantly shorter in the US group compared to the CT group (7 vs. 17.5, P < 0.0001). Our hypothesis for this difference is that in a center with a patient population at high risk for lung cancer, the significance of a lung lesion for a pulmonologist is considerably more urgent, as it is within a pulmonologists scope of primary specialty, as than for an interventional radiologist. Furthermore, a pulmonologist, besides performing the diagnostic procedure, also coordinates the postprocedure care of the patient with outpatient follow-up. One can argue that this was a single center study and reflects the practice pattern in our institution. The potential cancer diagnosis can be very anxiety provoking for a patient and. Therefore, the expediency with which a diagnosis can be made can be comforting to them. To optimize patient care one should adopt practice patterns that enhance the same.
Besides the advantage of real-time guidance, there is no radiation exposure with US-guided procedures. Patients undergoing CT-guided biopsy are exposed to radiation, and it does not offer real-time guidance. Although CT fluoroscopy allows one to perform the biopsy with real-time guidance, it provides only transverse sections and subject's patients to high radiation exposure.[4] US machines are small and portable, allowing them to be brought to a patient's bedside if need be, and the procedure performed in a position comfortable to the patient. CT scans, on the other hand, are usually situated in radiology suites. Hence, the patient has to be taken to where it is located, thereby becoming a limiting factor for critically ill patients. A patient with dyspnea in prone or supine position will not be able to tolerate a CT-guided biopsy.
Increasingly, minimally invasive procedures are performed to assess lung lesions and stage lung carcinomas. In cases of advanced-stage lung cancer, there is a concern that the biopsy may provide the only diagnostic tissue and may not be optimal for providing sufficient tissue for rendering a specific diagnosis and pursuing molecular studies for guiding tumor-specific treatment. In their study, Coley et al.[20] showed that FNAB, core biopsy or both techniques were comparable for arriving at a specific diagnosis and having sufficient tissue for molecular studies. The presence of an on-site cytopathologist is very helpful, as the proceduralist can communicate with the cytopathologist during the procedure regarding the adequacy of material for diagnosis and molecular studies. We did have one false positive case for malignancy in the US-guided group in our study, despite an on-site pathologist being confident of the adequacy of material obtained with FNAB. An internal and external peer-review conducted later did show that the presence of highly reactive Type II pneumocytes in the FNAB sample, which can mimic malignancy, made it difficult to distinguish them from malignant cells.[21]
Although, we did not perform a cost based analysis, US machine is considerably less expensive as compared to a CT scanner and given the rise in the use of point-of-care US, it is readily available in most centers.[12,22,23] We would like to emphasize that CT-guided biopsies are traditionally performed by interventional radiologists in most centers while US-guided biopsies besides them, are also done by pulmonologists.[5,15] Interventional radiology is a highly specialized field, usually limited to tertiary centers while pulmonary specialists are available in most centers. Granted, the number of pulmonologists currently performing US-guided biopsies is few, but in our view this technique can be taught and learned easily, thus allowing for a wider availability of US-guided biopsies for peripheral lung lesions in the future.
CT-guided biopsy had some advantages over US-guided biopsies for pleural-based lung lesions. CT provides excellent spatial resolution compared to US. US-guided biopsy requires the presence of contiguous nonaerated tissue between the lesion and the patient's chest wall and is not feasible if a pneumothorax develops during the procedure. Samples can be obtained with CT-guided biopsy in the event a small pneumothorax develops during the procedure, as the lesion can still be visualized and the needle guided to it. In addition, a good acoustic window may not be available with US if the contact area between the lesion and the pleura is small, but it may be feasible to guide the needle to such lesions under CT guidance.[24]
Several limitations of our study should be considered. Firstly, this is a retrospective chart review, and a patient selection bias cannot be excluded. Interventional radiologists performed the CT guided biopsies, and the US guided biopsy by pulmonologists based on referrals to their respective services. The patients were not randomized between the two groups, and we do not have the data as to how many patients referred to the pulmonary section for peripheral lung lesion were considered not suitable for US-guided biopsy. Although, the patient demographics and lesion size were not significantly different between the two groups. Patients with peripheral lung lesions that are behind the scapula, sternum, or ribs, will be difficult to visualize with US and hence unsuitable for US-guided biopsy. However, of those selected for US-guided biopsy, only one patient who had a negative result was later diagnosed to have a malignancy with a CT- guided biopsy. We do not have data on the rate and extent of emphysema in our patients, which could be a factor in the occurrence of pneumothorax. The number of needle punctures and the procedure time was not available in our retrospective review, as it was not documented in most procedures. This is a single-center study with a patient population that has a high likelihood of cancer, therefore, the results cannot be generalized to other settings. The presence of local expertise to perform US- and CT-guided biopsies, too will determine the generalizability of the results.
CONCLUSION
Our study shows that the diagnostic accuracy of US-guided biopsy of pleural-based lung lesion is similar to that of CT-guided biopsy, with a lower complication rate and a significantly reduced time to the procedure. US-guided biopsy can be considered as an alternate to CT-guided biopsy for pleural based lung lesions, provided local expertise is available, and the lesion is easily visualized and accessible with US guidance. We propose a larger randomized trial to confirm our conclusions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Oswald NC, Hinson KF, Canti G, Miller AB. The diagnosis of primary lung cancer with special reference to sputum cytology. Thorax. 1971;26:623–7. doi: 10.1136/thx.26.6.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e142S–65S. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]
- 3.Herman PG, Hessel SJ. The diagnostic accuracy and complications of closed lung biopsies. Radiology. 1977;125:11–4. doi: 10.1148/125.1.11. [DOI] [PubMed] [Google Scholar]
- 4.Kim GR, Hur J, Lee SM, Lee HJ, Hong YJ, Nam JE, et al. CT fluoroscopy-guided lung biopsy versus conventional CT-guided lung biopsy: A prospective controlled study to assess radiation doses and diagnostic performance. Eur Radiol. 2011;21:232–9. doi: 10.1007/s00330-010-1936-y. [DOI] [PubMed] [Google Scholar]
- 5.Diacon AH, Schuurmans MM, Theron J, Schubert PT, Wright CA, Bolliger CT. Safety and yield of ultrasound-assisted transthoracic biopsy performed by pulmonologists. Respiration. 2004;71:519–22. doi: 10.1159/000080638. [DOI] [PubMed] [Google Scholar]
- 6.Harter LP, Moss AA, Goldberg HI, Gross BH. CT-guided fine-needle aspirations for diagnosis of benign and malignant disease. AJR Am J Roentgenol. 1983;140:363–7. doi: 10.2214/ajr.140.2.363. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen OM, Aasen TB, Gulsvik A. Fine needle aspiration biopsy of mediastinal and peripheral pulmonary masses guided by real-time sonography. Chest. 1986;89:504–8. doi: 10.1378/chest.89.4.504. [DOI] [PubMed] [Google Scholar]
- 8.Yankelevitz DF, Cham MD, Farooqi AO, Henschke CI. CT-directed diagnosis of peripheral lung lesions suspicious for cancer. Thorac Surg Clin. 2007;17:143–58. doi: 10.1016/j.thorsurg.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasekhar AJ, Reynes CJ, Churchill RJ. Ultrasonically guided percutaneous biopsy of peripheral pulmonary masses. Chest. 1976;70:627–30. doi: 10.1378/chest.70.5.627. [DOI] [PubMed] [Google Scholar]
- 10.Liao WY, Chen MZ, Chang YL, Wu HD, Yu CJ, Kuo PH, et al. US-guided transthoracic cutting biopsy for peripheral thoracic lesions less than 3 cm in diameter. Radiology. 2000;217:685–91. doi: 10.1148/radiology.217.3.r00dc21685. [DOI] [PubMed] [Google Scholar]
- 11.Sconfienza LM, Mauri G, Grossi F, Truini M, Serafini G, Sardanelli F, et al. Pleural and peripheral lung lesions: Comparison of US- and CT-guided biopsy. Radiology. 2013;266:930–5. doi: 10.1148/radiol.12112077. [DOI] [PubMed] [Google Scholar]
- 12.Morris AE. Point-of-care ultrasound: Seeing the future. Curr Probl Diagn Radiol. 2015;44:3–7. doi: 10.1067/j.cpradiol.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Dodd GD, 3rd, Esola CC, Memel DS, Ghiatas AA, Chintapalli KN, Paulson EK, et al. Sonography: The undiscovered jewel of interventional radiology. Radiographics. 1996;16:1271–88. doi: 10.1148/radiographics.16.6.8946535. [DOI] [PubMed] [Google Scholar]
- 14.Turner EE, Fox JC, Rosen M, Allen A, Rosen S, Anderson C. Implementation and assessment of a curriculum for bedside ultrasound training. J Ultrasound Med. 2015;34:823–8. doi: 10.7863/ultra.34.5.823. [DOI] [PubMed] [Google Scholar]
- 15.Khosla R, Rohatgi PK, Seam N. Ultrasound-guided fine needle aspiration biopsy of pleural-based intrathoracic lesions. J Bronchology Interv Pulmonol. 2009;16:87–90. doi: 10.1097/LBR.0b013e31819b2dee. [DOI] [PubMed] [Google Scholar]
- 16.vanSonnenberg E, Casola G, Ho M, Neff CC, Varney RR, Wittich GR, et al. Difficult thoracic lesions: CT-guided biopsy experience in 150 cases. Radiology. 1988;167:457–61. doi: 10.1148/radiology.167.2.3357956. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Boiselle PM, Shepard JO, Trotman-Dickenson B, McLoud TC. Diagnostic accuracy and safety of CT-guided percutaneous needle aspiration biopsy of the lung: Comparison of small and large pulmonary nodules. AJR Am J Roentgenol. 1996;167:105–9. doi: 10.2214/ajr.167.1.8659351. [DOI] [PubMed] [Google Scholar]
- 18.Ikezoe J, Morimoto S, Arisawa J, Takashima S, Kozuka T, Nakahara K. Percutaneous biopsy of thoracic lesions: Value of sonography for needle guidance. AJR Am J Roentgenol. 1990;154:1181–5. doi: 10.2214/ajr.154.6.2110724. [DOI] [PubMed] [Google Scholar]
- 19.Sheth S, Hamper UM, Stanley DB, Wheeler JH, Smith PA. US guidance for thoracic biopsy: A valuable alternative to CT. Radiology. 1999;210:721–6. doi: 10.1148/radiology.210.3.r99mr23721. [DOI] [PubMed] [Google Scholar]
- 20.Coley SM, Crapanzano JP, Saqi A. FNA, core biopsy, or both for the diagnosis of lung carcinoma: Obtaining sufficient tissue for a specific diagnosis and molecular testing. Cancer Cytopathol. 2015;123:318–26. doi: 10.1002/cncy.21527. [DOI] [PubMed] [Google Scholar]
- 21.Idowu MO, Powers CN. Lung cancer cytology: Potential pitfalls and mimics – A review. Int J Clin Exp Pathol. 2010;3:367–85. [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson D, Arntfield RT. Critical care ultrasonography. Emerg Med Clin North Am. 2014;32:907–26. doi: 10.1016/j.emc.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Soni NJ, Lucas BP. Diagnostic point-of-care ultrasound for hospitalists. J Hosp Med. 2015;10:120–4. doi: 10.1002/jhm.2285. [DOI] [PubMed] [Google Scholar]
- 24.Jeon KN, Bae K, Park MJ, Choi HC, Shin HS, Shin S, et al. US-guided transthoracic biopsy of peripheral lung lesions: Pleural contact length influences diagnostic yield. Acta Radiol. 2014;55:295–301. doi: 10.1177/0284185113494984. [DOI] [PubMed] [Google Scholar]


