Fig. 2.
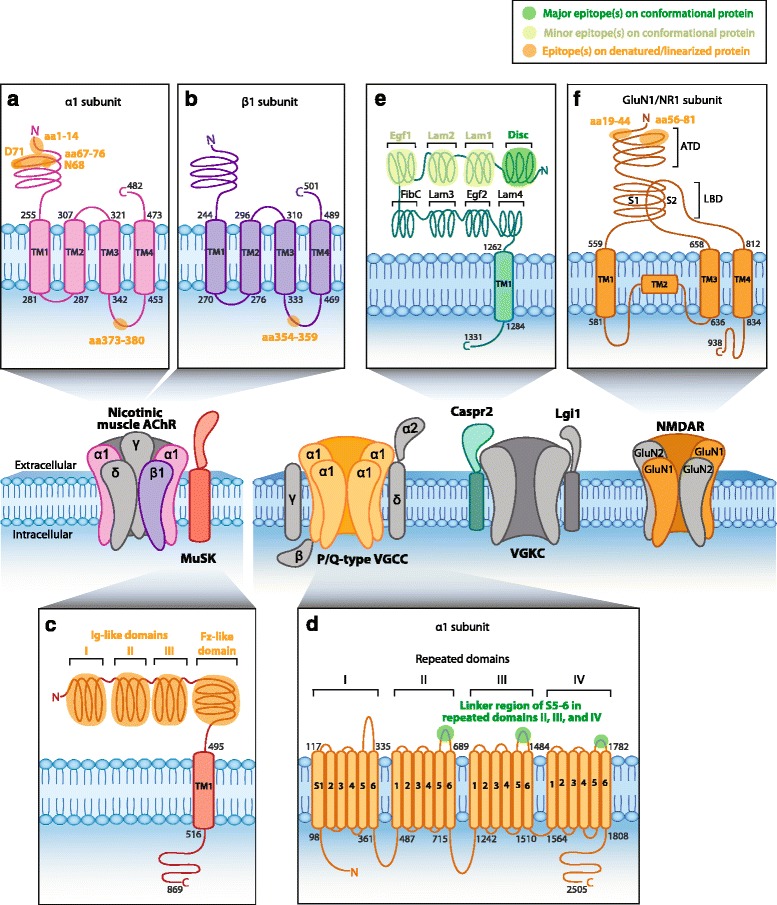
Epitopes of human PNS autoantibodies. Nicotinic muscle acetylcholine receptor (AChR) and muscle-specific kinase (MuSK, O15146) are postsynaptic muscle proteins. Anti-AChR antibodies target an extracellular epitope on the α1 subunit within amino acids (aa)1-14 [79] and aa67-76 [33]. Cytoplasmic epitopes have also been mapped in the α1 subunit at aa373-380 (a, P02708) and aa354–359 in the β1 subunit (b, P11230) [82]. c MuSK contains three immunoglobulin (Ig)-like domains and a Frizzled (Fz)-like domain. Anti-MuSK antibodies bind to the three N-linked Ig-like domains and the Fz-like domain [83–85]. d The neuronal P/Q-type voltage-gated calcium channel (VGCC) consists of four repeated domains, each containing six segments (S), with a linker region between S5 and S6. Anti-P/Q-type VGCC antibodies recognize epitopes in the linker region of repeating domains II–IV [94–96]. e Neuronal anti-contactin-associated protein 2 (Caspr2, Q9UHC6) antibodies recognize the extracellular N-terminal half (domains I–IV) but most commonly bind to an epitope within the discoidin (Disc) domain [37, 38]. f Neuronal N-methyl-D-aspartate receptor (NMDAR) contains an extracellular amino terminal domain (ATD) and a ligand-binding domain (LBD) formed by segment 1 (S1) and segment 2 (S2). Anti-NMDAR antibodies recognize aa19-44 and aa56-81 in the ATD of the GluN1 subunit (f, Q05586) [72]. Human protein topology, i.e., aa sequences and transmembrane domains (TM), is adapted from UniProt database, and UniProt identifiers are shown between brackets. Diagrams do not depict protein crystal structure. Green highlights major (dark green) and minor (light green) epitopes mapped by methods which retain the native in vivo conformational structure of the protein, such as cell-based assays. Orange highlights epitopes determined by methods which denature protein, such as western blots and ELISAs (Table 1)
