Abstract
The FOXO1 (forkhead box O1) transcription factor influences many key cellular processes, including those important in metabolism, proliferation and cell death. Reversible phosphorylation of FOXO1 at Thr24 and Ser256 regulates its subcellular localization, with phosphorylation promoting cytoplasmic localization, whereas dephosphorylation triggers nuclear import and transcriptional activation. In the present study, we used biochemical and molecular approaches to isolate and link the serine/threonine PP2A (protein phosphatase 2A) holoenzyme containing the B55α regulatory subunit, with nuclear import of FOXO1 in pancreatic islet β-cells under oxidative stress, a condition associated with cellular dysfunction in Type 2 diabetes. The mechanism of FOXO1 dephosphorylation and nuclear translocation was investigated in pancreatic islet INS-1 and βTC-3 cell lines subjected to oxidative stress. A combined chemical cross-linking and MS strategy revealed the association of FOXO1 with a PP2A holoenzyme composed of the catalytic C, structural A and B55α regulatory subunits. Knockdown of B55α in INS-1 cells reduced FOXO1 dephosphorylation, inhibited FOXO1 nuclear translocation and attenuated oxidative stress-induced cell death. Furthermore, both B55α and nuclear FOXO1 levels were increased under hyperglycaemic conditions in db/db mouse islets, an animal model of Type 2 diabetes. We conclude that B55α-containing PP2A is a key regulator of FOXO1 activity in vivo.
Keywords: B55α regulatory subunit, diabetes, forkhead box O1 (FOXO1), oxidative stress, pancreatic β-cell, protein phosphatase 2A (PP2A)
INTRODUCTION
The FOXO (forkhead box O) transcription factors control many fundamental cellular processes, including glucose and lipid metabolism, cell proliferation, cell-cycle arrest, apoptosis and signalling of other cellular stresses [1–3]. Four distinct FOXO isoforms have been identified in mammals: FOXO1 [FKHR (fork-head in rhabdosarcoma)], FOXO3 [FKHRL1 (FKHR-like 1)], FOXO4 [AFX (acute lymphocytic leukaemia 1-fused gene from chromosome X)] and FOXO6. FOXO proteins shuttle between the cytoplasm and the nucleus as a result of reversible phosphorylation. Akt/PKB (protein kinase B) phosphorylation of FOXO1 at Thr24, Ser256 and Ser319 drives export from the nucleus to the cytoplasm, whereas dephosphorylation regulates nuclear import [4–6]. Although many kinases have been associated with this process, little is known about the phosphatases controlling FOXO protein subcellular redistribution.
PP2A (protein phosphatase 2A) is important to the activity of pancreatic islet β-cells [7], which uniquely secrete the hormone insulin in response to elevated blood glucose levels to regulate glucose homoeostasis and metabolic balance. Oxidative stress contributes to both β-cell dysfunction and reduced cell mass in Type 2 diabetes [8–11]. Emerging evidence indicates that PP2A-catalysed FOXO1 dephosphorylation contributes to nuclear translocation and activation in β-cell lines and islet β-cells of Type 2 diabetes rodent models [12–16].
The PP2A holoenzymes are ubiquitously expressed serine/ threonine phosphatases, each consisting of a catalytic C subunit, a structural scaffolding A subunit and a variable B regulatory subunit. Subunits A and C form a stable core dimer. There are just two isoforms (α and β) of the catalytic subunit C and scaffolding subunit A, with α isoforms being much more abundant than β isoforms. In contrast, there are four B subunit families (B, B′, B″ and B‴), each consisting of several members encoded by distinct genes [17–19], which together amount to a large number of B subunits. The multiple isoforms of the regulatory B subunits give rise to the diversity of PP2A holoenzymes. Whereas the A and C subunits are ubiquitously expressed, the B subunits are more specific to tissue and cell type or developmental stage. The dynamic interaction of the B subunits with the core AC dimer contributes to the target specificity and subcellular localization of individual PP2A holoenzymes [20–22]. Our previous studies have shown that PP2A regulates FOXO1 subcellular localization in response to cell death stimuli [23]. However, the crucial question of which regulatory B subunit is targeting PP2A to FOXO1 remains largely unanswered. In the present study, we investigated the role of PP2A in oxidative signalling in a diabetic model and demonstrated that the B55α subunit regulates PP2A-catalysed FOXO1 dephosphorylation and nuclear translocation in pancreatic β-cells.
EXPERIMENTAL
Reagents
The following antibodies were used. For Western blots: phospho-Thr24-FOXO1, phospho-Ser256-FOXO1, phospho-Ser473-Akt, Akt and PP2A/A subunit (Cell Signaling Technology); PP2A/C subunit (BD Biosciences); and FOXO1 (H-128 and N-18) and PP2A/B55α (2G9) (Santa Cruz Biotechnology). For immunohistochemistry: insulin (Jackson ImmunoResearch), PP2A/B55α (2G9) and PP2A/B56α (C-19) (Santa Cruz Biotechnology); Rbbp5 (retinoblastoma-binding protein 5) (ab84511, Abcam), PABP [poly(A)-binding protein] (ab21060, Abcam), KAP1 (Krüppel-associated box zinc-finger protein 1) (ab10438, Abcam); Pdx1 (pancreatic and duodenal homeobox 1) (BCBC Consortium); FOXO1 (C29H4) (Cell Signaling Technology); and Cy3 (indocarbocyanine)-conjugated anti-(rabbit IgG), Cy3-conjugated anti-(mouse IgG), Cy3-conjugated anti-(goat IgG), and Cy2 (carbocyanine)-conjugated anti-(guinea pig IgG) (Jackson ImmunoResearch). PP2A/B55α siRNA (small interfering RNA) and scrambled siRNA were purchased from Santa Cruz Biotechnology, anti-HA (haemagglutinin)–agarose and anti-FLAG M2 affinity gel were from Sigma–Aldrich, and Colloidal Blue kit was from Invitrogen. The pcDNA3-GFP-FOXO1, pcDNA3-FLAG-FOXO1 and pcDNA3-HA-FOXO1 plasmids were kindly provided by Dr William R. Sellers (Harvard Medical School, Boston, MA, U.S.A.).
Cell lines and cultures
Rat insulinoma INS-1 cells were cultured in RPMI 1640 medium containing 11 mM glucose supplemented with 10 % (v/v) fetal bovine serum, 10 mM Hepes, 1 mM sodium pyruvate and 0.05 mM 2-mercaptoethnaol. Mouse islet β-cell-derived βTC-3 and HEK (human embryonic kidney)-293 cells were grown in DMEM (Dulbecco’s modified Eagle’s medium) containing 10 % (v/v) fetal bovine serum.
Immunohistochemical analysis of pancreatic islets and β-cell lines
Tissue fixation, embedding and immunofluorescence labelling were performed as described previously [24]. Briefly, pancreatic tissues from 10-week-old db/db and wild-type mice were fixed with 4 % (w/v) paraformaldehyde for 3 h on ice and then embedded in paraffin. Pancreatic sections were incubated with rabbit anti-FOXO1 (1:300), anti-PP2A/B55α (1:100), anti-PP2A/B56α (1:100), anti-Rbbp5 (1:500), anti-PP2A/C (1:500), anti-PABP (1:1000), anti-KAP1 (1:1000) or anti-Pdx1 (1:10000), or guinea pig anti-insulin (1:2000) antibodies at the dilutions indicated in parentheses. Immune complexes were detected using Cy2-conjugated anti-(guinea pig IgG) (1:1000), Cy3-conjugated anti-(rabbit IgG) (1:1000), Cy3-conjugated anti-(mouse IgG) (1:1000) or Cy3-cojugated goat-(rabbit IgG) (1:1000) antibodies at the dilutions indicated in parentheses. The images were visualized using a Zeiss Imager M2 microscope.
βTC-3 or INS-1 cells were cultured on 22 mm×22 mm coverslips in six-well plates for 24 h. Cells were incubated with 50 μM hydrogen peroxide (H2O2) for 60 min, fixed with 4% (w/v) paraformaldehyde for 12 min and permeabilized with 0.5% Triton X-100 for 5 min at room temperature (25°C). FOXO1 localization was detected with rabbit anti-FOXO1 antibody and nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole). Images were taken with a Zeiss Imager M2 microscope.
IP (immunoprecipitation) and immunoblotting
Cell lysates for Western blotting were prepared in RIPA (radioimmunoprecipitation assay) buffer (100 mM NaCl, 1% Nonidet P40, 0.5% deoxycholic acid, 0.1% SDS, 50 mM Tris/HCl, pH 8.0, and 1 mM EDTA). For IP, cells were lysed in isotonic IP buffer (142.5 mM KCl, 5 mM MgCl2, 10 mM Hepes and 0.25% Nonidet P40) with protease inhibitors and incubated with anti-HA–agarose or anti-FLAG M2 affinity gel. HA–FOXO1 or FLAG–FOXO1 complexes precipitated with affinity agarose were fractionated by SDS/PAGE (12.5% gels), and transferred on to PVDF membranes. Blots were probed with the indicated antibodies.
Cross-linking and MS identification of FOXO1-associated proteins
The pcDNA3-HA-FOXO1 expression plasmid was transfected into HEK-293 cells. Whole-cell extracts of untransfected and HA–FOXO1-transfected cells prepared in IP buffer 48 h after transfection were incubated with 2 mM DTSSP [3,3′-dithiobis(sulfosuccinimidylpropionate)] cross-linker (Thermo Scientific) at 4°C for 2 h. Cross-linking reactions were quenched by adding Tris/HCl to a final concentration of 20 mM (pH 7.5). The cross-linked samples were incubated with anti-HA–agarose for 2 h at 4 °C, and washed with RIPA buffer. The HA–agarose pull-downs were boiled in 2× gel loading buffer and separated by SDS/PAGE (12.5% gels). Then, 20% of the samples were separated by SDS/PAGE for silver staining and 80% were separated by SDS/PAGE with Colloidal Blue staining for MS. The improved silver staining method was used. Briefly, the polyacrylamide gel was fixed in 40% ethanol, 12% acetic acid and 0.02% formaldehyde overnight, washed with 50% ethanol three times, then treated with 0.02% sodium thiosulfate for 1 min. The gel was then rinsed in ultrapure water three times for 20 s each and impregnated in staining buffer (0.2% silver nitrate and 0.03% formaldehyde) for 20 min. Next, the gel was incubated in developing solution (6% sodium carbonate, 0.02% formaldehyde and one drop of 10% sodium thiosulfate) for 2–5 min until the desired band intensity was reached. The reaction was stopped by immersing the gel in 12% acetic acid. For Colloidal Blue-stained gels, sections were excised to avoid the Ig heavy chain and light chain, which were separately excised and analysed. Excised gel sections from both the control and HA–FOXO1 lanes were submitted to the Vanderbilt Proteomics Laboratory for in-gel tryptic digest and analysis by C18 reverse-phase liquid chromatography–MS/MS (tandem MS) using a Thermo LTQ ion-trap mass spectrometer equipped with a Thermo MicroAS autosampler and Eksigent HPLC nanoLC pump system, nanospray source and Xcalibur 2.0 instrument control. MS/MS data were analysed with the Sequest algorithm. Peptides from the control sample were subtracted from the HA–FOXO1 sample, and the remaining peptides were considered to be specific to the HA–FOXO1 interaction.
PP2A/B55α subunit knockdown and nuclear translocation assays
INS-1 cells were co-transfected with pcDNA3-GFP-FOXO1 and PP2A/B55α siRNA or scrambled siRNA using the Invitrogen Lipofectamine™ 2000 kit. FOXO1 and PP2A/B55α levels were assayed 30–48 h after transfection by Western blotting. Transfected cells were treated with 100 μM H2O2 for 1 h, and subcellular GFP (green fluorescent protein) localization was assessed by fluorescence microscopy. Cell death was measured in untreated and H2O2-treated cells using the propidium iodide exclusion assay, analysed by flow cytometry [23].
RESULTS
Oxidative stress induces FOXO1 dephosphorylation at Thr24 and Ser256 and nuclear translocalization
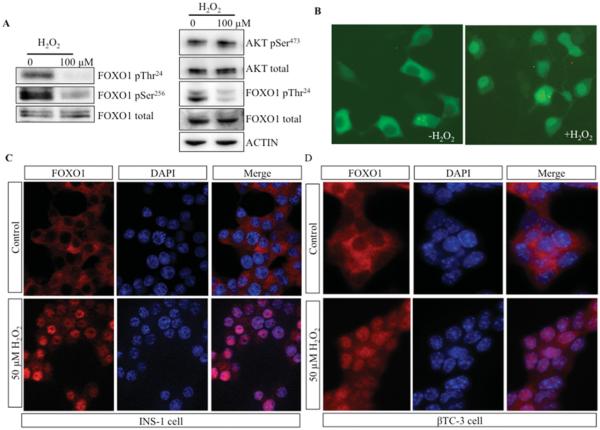
Since β-cell dysfunction has been related to hyperglycaemia-induced oxidative stress, we used H2O2 as an oxidative stressor to investigate FOXO1 dephosphorylation and localization in pancreatic β-cell lines. FOXO1 dephosphorylation after H2O2 treatment has been reported previously in HEK-293 cells [25]. We determined the phosphorylation states of Thr24 and Ser256 in rat insulinoma INS-1 cells following H2O2 treatment. Steady-state phosphorylation levels of FOXO1 Thr24 and Ser256 were reduced significantly by Western blot analysis (Figure 1A, left-hand panel). The activity of the FOXO1 kinase Akt is also regulated by phosphorylation events, one of which is phospho-Ser473 [26]. The phosphorylation status of Akt Ser473 did not change appreciably following H2O2 treatment, suggesting that decreased FOXO1 phosphorylation resulted from dephosphorylation and not reduced Akt kinase activity (Figure 1A, right-hand panel). Consistent with FOXO1 dephosphorylation, GFP–FOXO1 was relocalized to the nucleus after H2O2 treatment (Figure 1B). Immunostaining showed that endogenous FOXO1 also translocated into the nucleus of H2O2-treated β-cells, including INS-1 and βTC-3 cells (Figures 1C and 1D). Not unexpectedly, our results suggest a link between FOXO1 phosphorylation levels and subcellular localization in β-cells under oxidative stress.
Figure 1. FOXO1 was dephosphorylated and translocated into the nucleus in H2O2-treated islet β-cell lines.
(A) INS-1 cells were untreated or treated with 100 μM H2O2 for 1 h, and lysates were immunoblotted for FOXO1 phosphorylated (p) at Thr24 or Ser256 and total FOXO1 (left-hand panel), or Akt phosphorylated at Ser473 and total Akt, FOXO1 phosphorylated at Thr24 and total FOXO1, and actin (right-hand panel). (B) Representative micrographs of GFP–FOXO1 fluorescence in INS-1 cells treated or not treated with 100 μM H2O2 . The localization of endogenous FOXO1 was determined by immunohistochemistry in INS-1 (C) and βTC-3 (D) cells treated with 50 μM H2O2 for 90 min. DAPI staining served as a nuclear marker (C and D).
B55α and PP2A/AC specifically interact with FOXO1
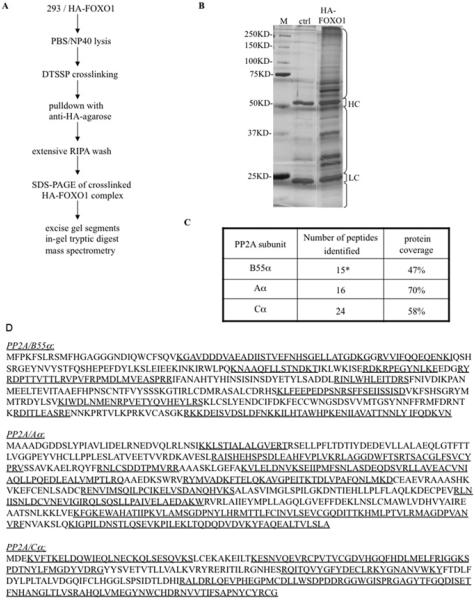
The catalytic and structural subunits of PP2A have been shown to interact with FOXO1, but the specific regulatory B subunit targeting PP2A to FOXO1 was not identified [23]. We used a combined cross-linking and MS strategy to determine the complete composition of the PP2A holoenzyme that dephosphorylates FOXO1. Cross-linking was performed in untransfected and HA–FOXO1-transfected HEK-293 whole-cell extracts using the cross-linker DTSSP, which targets protein amino groups (Figure 2A). Cross-linked lysates from untreated cells and cells treated with the apoptosis stimulator STS (staurosporine) were incubated with anti-HA–sepharose. Precipitated proteins were analysed by MS (Figure 2B). Peptides matching the PP2A catalytic (PP2A/C) and structural (PP2A/A) subunits were only recovered from the HA–FOXO1 and not the control immunocomplex (results not shown). Additionally, peptides matching the B55α regulatory subunits were detected in the HA–FOXO1 complex. The proportions of the cognate protein sequence covered by the peptides were robust at 47, 70 and 58% of B55α, PP2A/A and PP2A/C respectively (Figures 2C and 2D). No other regulatory B subunit peptides were detected in the HA–FOXO1 complex from STS-treated cells.
Figure 2. B55α was identified by MS in the PP2A/C complex cross-linked to FOXO1.
(A) The strategy used to purify and identify DTSSP-cross-linked HA–FOXO1 proteins is outlined. NP40, Nonidet P40. (B) Colloidal Blue staining of anti-HA affinity gel-precipitated proteins from untransfected HEK-293 [control (ctrl)] or HA–FOXO1-transfected HEK-293 cells (HA–FOXO1) treated with STS. Brackets show the regions of the lanes excised and analysed separately to avoid interference by Ig heavy chain (HC) and light chain (LC). Molecular masses are indicated in kDa. (C) The number of peptides identified by MS from the HA–FOXO1 sample, after subtracting the control, and percentage coverage of each protein. *12 peptides were specific to B55α, three peptides were common to B55α, B55γ, B55β and B55δ. No PP2A peptides were found in the control sample. (D) Tryptic peptides identified by MS are underlined in the amino acid sequence of each subunit of human PP2A.
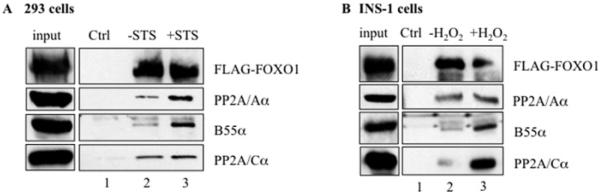
To determine whether the FOXO1–PP2A/AB55αC complex could be detected in cells, INS-1 and HEK-293 cells were transfected with FLAG–FOXO1. The presence of the PP2A subunits was analysed in anti-FLAG immunoprecipitates by Western blotting (Figure 3). All three PP2A subunits, A, C and B55α, were associated with FLAG–FOXO1. Moreover, the level of the PP2A holoenzyme complex recruited by FOXO1 was increased upon exposure to STS in HEK-293 cells or to H2O2 in INS-1 cells (Figures 3A and 3B, compare lanes 2 and 3). Thus we have identified B55α as the specific regulatory subunit in the PP2A holoenzyme associated with FOXO1, and showed that recruitment is mediated by exposure to an apoptotic or oxidative stimulus.
Figure 3. PP2A subunits C, A and B55α associated with FOXO1.
FLAG–FOXO1 was transfected into HEK-293 cells (A) and INS-1 cells (B). Proteins precipitated by anti-FLAG–agarose before and after treatment of (A) HEK-293 cells with 2 μM STS for1 h or (B) INS-1 cells with 100 μM H2O2 for 1 h were analysed for FLAG–FOXO1, and the PP2A subunits A, C and B55α by Western blot analysis. Sepharose beads alone were incubated with lysates from transfected cells as control (Ctrl). Portions of the lysates were loaded as ‘input’.
FOXO1 phosphorylation level and nuclear distribution is regulated by PP2A/AB55αC
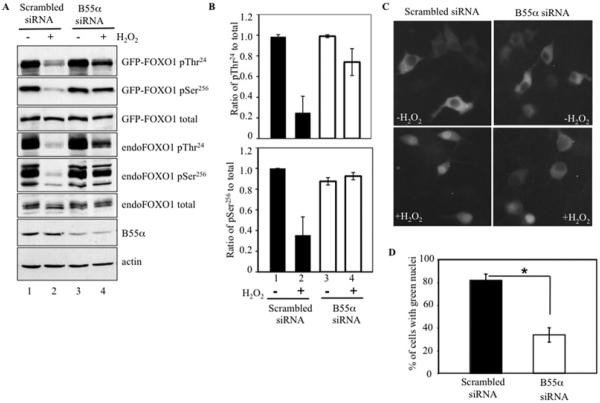
To determine the functional significance of the association of FOXO1 with B55α, FOXO1 phosphorylation state was analysed in INS-1 cells with reduced B55α protein levels (Figure 4). Transfection of INS-1 cells with B55α siRNA led to a greater than 50% reduction of B55α protein in immunoblots, when compared with scrambled siRNA (Figure 4A, compare lanes 1 and 2 with lanes 3 and 4), but B55α siRNA did not alter the phosphorylation levels of FOXO1 Thr24 or Ser256 in the absence of H2O2 treatment (Figure 4A, lanes 1 and 3). However, the phosphorylation level of Thr24 was reduced by 75% and that of Ser256 was reduced by 65% both in transfected GFP–FOXO1 and endogenous FOXO1 after scrambled siRNA and H2O2 treatment, whereas total FOXO1 level remained constant (Figures 4A and 4B, lanes 1 and 2). In contrast, there was only a 25% reduction in FOXO1 phospho-Thr24 and no change in phospho-Ser256 level in cells transfected with B55α siRNA and treated with H2O2 (Figures 4A and 4B, lanes 3 and 4). This result demonstrated a crucial role of B55α in FOXO1 dephosphorylation in oxidatively stressed INS-1 cells.
Figure 4. B55α knockdown inhibited oxidative stress-induced FOXO1 dephosphorylation and nuclear translocation.
(A) INS-1 cells were co-transfected with GFP–FOXO1 and B55α siRNA or scrambled siRNA, and immunoblotted for GFP–FOXO1 and endogenous FOXO1 using antibodies against phosphorylated (p) Thr24 and Ser256 and total FOXO1, as well as against B55α. Cells were incubated in the presence (+) or absence (−) of 100 μM H2O2 for 1 h. GFP–FOXO1 (97 kDa) is distinguished from endogenous FOXO1 (70 kDa) by its slower migration. (B) Ratios of phospho-Thr24 and phospho-Ser256 phosphorylated FOXO1 relative to total FOXO1 in untreated (−) or H2O2-treated (+) cells transfected with scrambled or B55α siRNA. Ratios were normalized to untreated cells transfected with scrambled siRNA. Band intensities were acquired from (A) using Bio-Rad quantity analysis software and represent the means±S.D. of four samples. (C) INS-1 cells were co-transfected with GFP–FOXO1 and B55α siRNA or scrambled siRNA. GFP localization was visualized by fluorescence microscopy in untreated and H2O2-treated cells. (D) Percentages of GFP-positive nuclei after H2O2 treatment in scrambled siRNA or B55α siRNA-transfected cells. At least 150 cells were counted in three independent experiments. *P <0.01.
To determine whether the B55α regulatory subunit also affected FOXO1 nuclear translocation, the subcellular localization of GFP–FOXO1 was determined in INS-1 cells transfected with B55α or scrambled control siRNA and then treated with H2O2 . Roughly 82% of scrambled siRNA cells showed green nuclear fluorescence, whereas only 34% of cells transfected with B55α siRNA contained nuclear GFP–FOXO1 (Figures 4C and 4D). Thus GFP–FOXO1 was preferentially retained in the cytoplasm in B55α-knockdown cells. Collectively, these results demonstrate that PP2A/AB55αC catalyses FOXO1 dephosphorylation at Thr24 and Ser256, allowing nuclear translocation in stressed islet β-cells.
B55α knockdown rescues oxidative stress-induced β-cell death
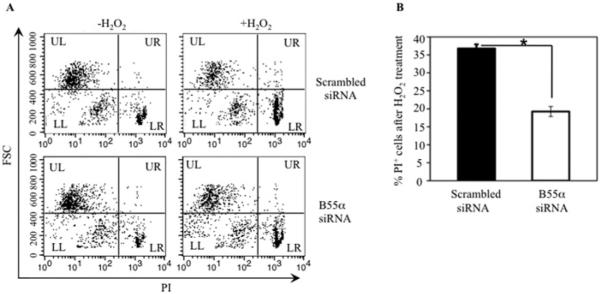
FOXO1 functions in multiple contexts, including transcription of pro-apoptotic genes as well as induction of antioxidant enzymes. We next analysed whether reducing B55α levels, and consequently PP2A-mediated FOXO1 dephosphorylation, would also reduce H2O2-induced INS-1 cell death. Cell viability was assayed by propidium iodide exclusion after B55α or scrambled siRNA transfection and H2O2 treatment. INS-1 cell death was measured by flow cytometry as low FSC (forward scatter) and high propidium iodide fluorescence (PI +) in the lower right (LR) quadrant (Figure 5A). Cell death was lowered by approximately 50% in B55α siRNA compared with scrambled siRNA transfected cultures (Figure 5B). Hence decreasing B55α expression significantly enhanced the survival of INS-1 cells under oxidative stress conditions, indicating a pro-apoptotic role for B55α.
Figure 5. B55α knockdown rescues H2O2-induced cell death.
(A) Untreated or H2O2-treated INS-1 cells transfected with B55α siRNA or scrambled siRNA were incubated with propidium iodide (PI), and analysed for PI exclusion by flow cytometry. Representative data from three experiments are shown. Small cells that failed to exclude PI (low FSC, high PI +) in the quadrant labelled LR (lower right) were scored as dead cells. (B) Mean±S.D. percentages of dead cells from three experiments in (A). n = 3, *P < 0.01.
FOXO1 is nuclear and B55α increased in islet β-cells of diabetic db/db mice
FOXO1 plays an essential regulatory role in the maintenance of pancreatic β-cell mass and function, in part through changes in its subcellular localization. Chronic hyperglyacemia causes oxidative stress, which can lead to β-cell dysfunction [27–29]. The leptin receptor-deficient db/db mice develop hyperglycaemia, with plasma glucose reaching a plateau approximately 10–12 weeks after birth. The diabetic phenotype of the db/db mice is reversible by β-cell-specific transgenic overexpression of the antioxidant enzyme glutathione peroxidase-1, demonstrating the importance of oxidative damage in this model of Type 2 diabetes [30,31].
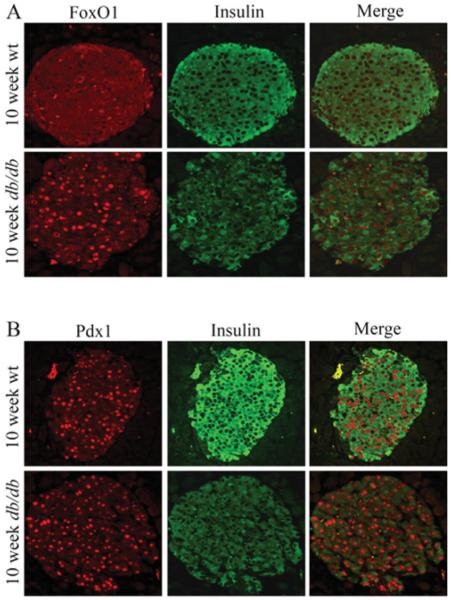
We first examined whether the subcellular site of FOXO1 was affected in 10-week-old diabetic db/db mice. FOXO1 was principally cytoplasmic in wild-type non-diabetic mice, yet nuclear in db/db islet cells (Figure 6A). This finding is consistent with previous studies illustrating that FOXO1 is translocated into the nucleus under H2O2-induced oxidative stress in βTC-3 cells and primary islets [32,33]. In contrast, Pdx1, an essential β-cell transcription factor, was only found in the nucleus of wild-type and db/db islets (Figure 6B). As expected, insulin levels were lower in db/db islets (Figure 6).
Figure 6. FOXO1 was translocated into the nucleus of islet β-cells in db/db diabetic mice.
Dual immunohistochemistry was performed in paraffin-embedded pancreatic sections from 10-week-old wild-type (wt) and db/db mice, comparing FOXO1 (red) and insulin (green) expression in (A), and Pdx1 (red) and insulin (green) expression in (B).
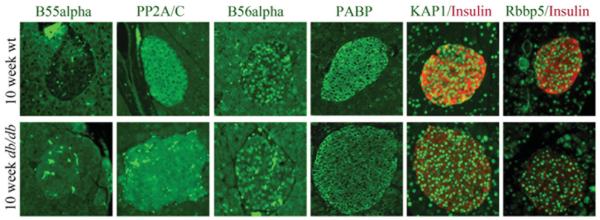
Since PP2A/AB55αC was found to catalyse FOXO1 dephosphorylation and nuclear translocation in INS-1 cells (Figure 4), we investigated whether the levels of PP2A/C and B55α were different in 10-week-old non-diabetic wild-type and diabetic db/db islet cells. Interestingly, B55α levels by immunostaining were very low in wild-type islets relative to surrounding acinar cells, but the B55α signal in db/db islets was much stronger and was comparable with that in the acinar cells (Figure 7). In support of the specificity of the increase in B55α, the signal intensities of PP2A/C and another regulatory subunit B56α were similar between wild-type and db/db islets under these conditions, a pattern also observed for PABP, KAP1 and Rbbp5, a common subunit in the histone H3 lysine 4 methyltransferase nuclear MLL (mixed lineage leukaemia) coactivator complex (Figure 7). In summary, the increase in B55α levels concomitant with FOXO1 redistribution to the nucleus in db/db islet β-cells provides compelling support for the PP2A/AB55αC holoenzyme regulating FOXO1 activity under oxidative stress conditions associated with Type 2 diabetes in vivo.
Figure 7. B55α levels were increased in the islet β-cells of db/db diabetic mice.
Dual immunohistochemistry was performed in paraffin-embedded pancreatic sections from 10-week-old wild-type and diabetic db/db mice, comparing PP2A/C (green), B55α (green), B56α (green), PABP (green), KAP1 (green) and Rbbp5α (green). Insulin (red) staining was analysed with KAP1 and Rbbp5. Representative staining from wild-type and db/db islets from at least three independent animals is shown.
DISCUSSION
FOXO1 is constitutively expressed in pancreatic β-cells and plays a key role in the regulation of β-cell mass and function [34,35]. The activity of FOXO1 is profoundly influenced by post-translational modification mechanisms, with dephosphorylation at specific residues required for nuclear localization. We showed previously a role for PP2A in nuclear translocation and FOXO1-mediated apoptosis in haemopoietic cells, but the full composition of the PP2A holoenzyme was not characterized. The principal aims of the present study were to identify the regulatory B subunit of the PP2A holoenzyme required in this process, and to investigate the role of this enzyme complex in FOXO1 activity in pancreatic β-cells exposed to oxidative stress. We have shown that B55α regulates dephosphorylation of FOXO1 by PP2A, which is required for oxidative-stress-induced nuclear localization of FOXO1 in β-cells. In addition, we found that B55α was elevated in islet cells of diabetic db/db mice, which represent cells undergoing adaptive processes in response to hyperglycaemia and oxidative stress requiring nuclear FOXO1 activity.
The substrate specificity of PP2A holoenzyme is largely determined by the specific regulatory subunit [20–22]. We first used a proteomics approach to identify B55α as a PP2A regulatory subunit in STS-treated HEK-293 cells (Figure 2), and subsequently demonstrated the importance of this regulatory subunit in PP2A-mediated dephosphorylation and nuclear translocation of FOXO1 in INS-1 cells (Figure 4). A stimulus-induced increase in B55α association with FOXO1 suggested a dynamic regulatory response to cellular stresses leading to cell death (Figure 3). We considered the possibility that the decrease in FOXO1 phosphorylation observed could be due to reduced Akt kinase activity instead of dephosphorylation by PP2A. Two pieces of data strongly indicated that this is unlikely. First, H2O2 treatment did not significantly alter Akt phosphorylation, suggesting that H2O2 treatment did not change Akt activity (Figure 1A). Secondly, the phosphorylation levels of FOXO1 were the same between scrambled and B55α siRNA samples before H2O2 treatment (Figure 4A). These findings indicated that, in the context of these experiments, neither H2O2 treatment nor B55α knockdown significantly affected Akt phosphorylation of FOXO1.
Several groups have investigated the physiological function of FOXO1 in multiple metabolic pathways, usually focusing on FOXO1 downstream target gene profiles or its regulation in the nucleus [36,37]. Our experiments were designed to examine the FOXO1 dephosphorylation process, which leads to its translocation to the nucleus where it is transcriptionally active. We found B55α to be the major regulatory subunit mediating FOXO1 dephosphorylation in signalling pathways associated with oxidative and apoptotic stress. Although we concentrated our studies in β-cells, MS and IP results suggested that B55α is also the PP2A regulatory subunit involved in STS-induced cell death. Thus B55α could be the regulatory subunit for the PP2A activation of FOXO1 in response to a variety of different cellular stresses in multiple distinct cell types.
B55α is in one of four families of B subunits, each with several members that mediate substrate specificity and subcellular localization of PP2A holoenzymes. In the cellular models used in the present study, acute oxidative stress induced the interaction of B55α-containing PP2A with cytoplasmic FOXO1, leading to dephosphorylation of this transcription factor and translocation to the nucleus. Various mechanisms have been described for altering the interaction of PP2A with other key regulatory proteins exposed to oxidative stress. These include regulation of PP2A association with Bcl-xL by neuroprotectin D in H2O2-treated retinal pigment epithelial cells [38], release of PP2A from microtubules to bind and dephosphorylate tau protein [39] and activation of PP2A by p38 MAPK (mitogen-activated protein kinase) to bind and dephosphorylate ERK (extracellular-signal-regulated kinase) in cardiomyocytes [40]. Interestingly, PP2A also promoted autophagy in neuroblastoma cells via ectopic transfer of mitochondrially targeted B subunit PPP2R2B, or Bβ2, in response to H2O2 and t-butyl hydroperoxide treatment [41].
The expression pattern of B55α was compared with that of B56α and FOXO1 in 10-week-old non-diabetic control and diabetic pancreatic sections by immunohistochemistry. As expected, FOXO1 was cytoplasmic in wild-type control islets and nuclear in the dysfunctional β-cells of the db/db mouse (Figure 6). B55α levels were significantly increased in db/db islets (Figure 7). This contrasted with the immunostaining pattern of B56α, which did not appear to differ between control and diabetic islet cells. Moreover, B55α levels in INS-1 cells were also unchanged upon a relatively brief 1 h exposure to H2O2 (Figure 4A). Collectively, these results suggest that B55α expression is induced by the chronic hyperglycaemic conditions associated with diabetes, presumably involving signalling from reactive oxygen species. How B55α levels are increased in this context is unknown. In another context, elevation in intracellular calcium concentration induced by H2O2 treatment directly regulated the increase of another B subunit, PR70/B″, resulting in PP2A activation [42–46].
We believe that B55α may be required under the experimental conditions used in the present study for FOXO1 to protect β-cells against oxidative damage, through stimulating key transcriptional regulators, such as MafA and NeuroD1 [32]. In addition, B55α may also be important in regulating β-cell expansion in diabetic islets, either independently or through FOXO1. For example, PP2A/AB55αC was found recently to control FoxM1 (forkhead box M1) transcription factor activity [47], a key regulator of β-cell replication [48]. Future efforts should be focused on determining the significance of B55α in regulating β-cell activity in vivo under the stress-induced conditions associated with diabetes.
ACKNOWLEDGEMENTS
We thank Vanderbilt Proteomics Laboratory for their assistance in our MS experiments and data analysis. We are grateful to Dr Charles Lin (Vanderbilt University) for the use of microscopy equipment. We appreciate a critical reading of the paper by Dr Brian Wadzinski (Vanderbilt University) prior to submission.
FUNDING
This work was supported by the National Institutes of Health [grant number R01CA92498 (to E.Y.) and 5R01DK050203 (to R.S.)], a fellowship from Hope Street Kids Foundation (to L.Y.) and a fellowship from Juvenile Diabetes Research Foundation (to S.G.).
Abbreviations used
- Cy2
carbocyanine
- Cy3
indocarbocyanine
- DAPI
4′,6-diamidino-2-phenylindole
- DTSSP
3,3′-dithiobis(sulfosuccinimidyl-propionate)
- FKHR
forkhead in rhabdosarcoma
- FOXO
forkhead box O
- FSC
forward scatter
- GFP
green fluorescent protein
- HA
haemagglutinin
- HEK
human embryonic kidney
- IP
immunoprecipitation
- KAP1
Krüppel-associated box zinc-finger protein 1
- MS/MS
tandem MS
- PABP
poly(A)-binding protein
- Pdx1
pancreatic and duodenal homeobox 1
- PP2A
protein phosphatase 2A
- Rbbp5
retinoblastoma-binding protein 5
- RIPA
radioimmunoprecipitation assay
- siRNA
small interfering RNA
- STS
staurosporine
Footnotes
AUTHOR CONTRIBUTION
Ling Yan and Marie Brault performed the experiments for Figures 1–5. Shuangli Guo contributed to Figures 6 and 7. Ling Yan, Shuangli Guo, Elizabeth Yang and Roland Stein designed the experiments and wrote the paper. Elizabeth Yang, Roland Stein, Rizwan Hamid, Jamie Harmon and R. Paul Robertson supervised the project.
REFERENCES
- 1.Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO’s road. Sci. STKE. 2003;2003:RE5. doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- 2.Nakae J, Oki M, Cao Y. The FoxO transcription factors and metabolic regulation. FEBS Lett. 2008;582:54–67. doi: 10.1016/j.febslet.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol. Cell. Biol. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rena G, Prescott AR, Guo S, Cohen P, Unterman TG. Roles of the forkhead in rhabdomyosarcoma (FKHR) phosphorylation sites in regulating 14-3-3 binding, transactivation and nuclear targetting. Biochem. J. 2001;354:605–612. doi: 10.1042/0264-6021:3540605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao X, Gan L, Pan H, Kan D, Majeski M, Adam SA, Unterman TG. Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem. J. 2004;378:839–849. doi: 10.1042/BJ20031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol. Cell. Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kowluru A. Novel regulatory roles for protein phosphatase-2A in the islet β cell. Biochem. Pharmacol. 2005;69:1681–1691. doi: 10.1016/j.bcp.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and β-cell dysfunction. Endocr. Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J. Physiol. 2007;583:9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenzen S. Oxidative stress: the vulnerable β-cell. Biochem. Soc. Trans. 2008;36:343–347. doi: 10.1042/BST0360343. [DOI] [PubMed] [Google Scholar]
- 11.Kaneto H, Matsuoka TA, Katakami N, Kawamori D, Miyatsuka T, Yoshiuchi K, Yasuda T, Sakamoto K, Yamasaki Y, Matsuhisa M. Oxidative stress and the JNK pathway are involved in the development of type 1 and type 2 diabetes. Curr. Mol. Med. 2007;7:674–686. doi: 10.2174/156652407782564408. [DOI] [PubMed] [Google Scholar]
- 12.Glauser DA, Schlegel W. The emerging role of FOXO transcription factors in pancreatic β cells. J. Endocrinol. 2007;193:195–207. doi: 10.1677/JOE-06-0191. [DOI] [PubMed] [Google Scholar]
- 13.Storz P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid. Redox. Signaling. 2011;14:593–605. doi: 10.1089/ars.2010.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawamori D, Kaneto H, Nakatani Y, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J. Biol. Chem. 2006;281:1091–1098. doi: 10.1074/jbc.M508510200. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs WH, 3rd, Wright CV, White MF, Arden KC, Accili D. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J. Clin. Invest. 2002;110:1839–1847. doi: 10.1172/JCI200216857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitamura T, Kitamura YI, Kobayashi M, Kikuchi O, Sasaki T, Depinho RA, Accili D. Regulation of pancreatic juxtaductal endocrine cell formation by FoxO1. Mol. Cell. Biol. 2009;29:4417–4430. doi: 10.1128/MCB.01622-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lechward K, Awotunde OS, Swiatek W, Muszynska G. Protein phosphatase 2A: variety of forms and diversity of functions. Acta Biochim. Pol. 2001;48:921–933. [PubMed] [Google Scholar]
- 18.Janssens V, Goris J, Van Hoof C. PP2A: the expected tumor suppressor. Curr. Opin. Genet. Dev. 2005;15:34–41. doi: 10.1016/j.gde.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Chen Y, Zhang P, Jeffrey PD, Shi Y. Structure of a protein phosphatase 2A holoenzyme: insights into B55-mediated Tau dephosphorylation. Mol. Cell. 2008;31:873–885. doi: 10.1016/j.molcel.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strack S, Chang D, Zaucha JA, Colbran RJ, Wadzinski BE. Cloning and characterization of Bδ, a novel regulatory subunit of protein phosphatase 2A. FEBS Lett. 1999;460:462–466. doi: 10.1016/s0014-5793(99)01377-0. [DOI] [PubMed] [Google Scholar]
- 21.Lee TY, Lai TY, Lin SC, Wu CW, Ni IF, Yang YS, Hung LY, Law BK, Chiang CW. The B56γ 3 regulatory subunit of protein phosphatase 2A (PP2A) regulates S phase-specific nuclear accumulation of PP2A and the G1 to S transition. J. Biol. Chem. 2010;285:21567–21580. doi: 10.1074/jbc.M109.094953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCright B, Rivers AM, Audlin S, Virshup DM. The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J. Biol. Chem. 1996;271:22081–22089. doi: 10.1074/jbc.271.36.22081. [DOI] [PubMed] [Google Scholar]
- 23.Yan L, Lavin VA, Moser LR, Cui Q, Kanies C, Yang E. PP2A regulates the pro-apoptotic activity of FOXO1. J. Biol. Chem. 2008;283:7411–7420. doi: 10.1074/jbc.M708083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Artner I, Hang Y, Mazur M, Yamamoto T, Guo M, Lindner J, Magnuson MA, Stein R. MafA and MafB regulate genes critical to β-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiang L, Banks AS, Accili D. Uncoupling of acetylation from phosphorylation regulates FoxO1 function independent of its subcellular localization. J. Biol. Chem. 2010;285:27396–27401. doi: 10.1074/jbc.M110.140228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Cron P, Thompson V, Good VM, Hess D, Hemmings BA, Barford D. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol. Cell. 2002;9:1227–1240. doi: 10.1016/s1097-2765(02)00550-6. [DOI] [PubMed] [Google Scholar]
- 27.Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, Hiai H, Seino Y, Yamada Y. Hyperglycemia causes oxidative stress in pancreatic β-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999;48:927–932. doi: 10.2337/diabetes.48.4.927. [DOI] [PubMed] [Google Scholar]
- 28.Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet β cells in diabetes. J. Biol. Chem. 2004;279:42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 29.Robertson RP, Harmon JS. Diabetes, glucose toxicity, and oxidative stress: a case of double jeopardy for the pancreatic islet β cell. Free Radical Biol. Med. 2006;41:177–184. doi: 10.1016/j.freeradbiomed.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 30.Harmon JS, Bogdani M, Parazzoli SD, Mak SS, Oseid EA, Berghmans M, Leboeuf RC, Robertson RP. β-Cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology. 2009;150:4855–4862. doi: 10.1210/en.2009-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robertson RP, Harmon JS. Pancreatic islet β-cell and oxidative stress: the importance of glutathione peroxidase. FEBS Lett. 2007;581:3743–3748. doi: 10.1016/j.febslet.2007.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D. FoxO1 protects against pancreatic β cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Buteau J, Accili D. Regulation of pancreatic β-cell function by the forkhead protein FoxO1. Diabetes Obes. Metab. 2007;9(Suppl. 2):140–146. doi: 10.1111/j.1463-1326.2007.00782.x. [DOI] [PubMed] [Google Scholar]
- 34.Buteau J, Shlien A, Foisy S, Accili D. Metabolic diapause in pancreatic β-cells expressing a gain-of-function mutant of the forkhead protein Foxo1. J. Biol. Chem. 2007;282:287–293. doi: 10.1074/jbc.M606118200. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto H, Hribal ML, Lin HV, Bennett WR, Ward A, Accili D. Role of the forkhead protein FoxO1 in β cell compensation to insulin resistance. J. Clin. Invest. 2006;116:775–782. doi: 10.1172/JCI24967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J. Biol. Chem. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
- 37.McKinnon CM, Ravier MA, Rutter GA. FoxO1 is required for the regulation of preproglucagon gene expression by insulin in pancreatic αTC1–9 cells. J. Biol. Chem. 2006;281:39358–39369. doi: 10.1074/jbc.M605022200. [DOI] [PubMed] [Google Scholar]
- 38.Antony R, Lukiw WJ, Bazan NG. Neuroprotectin D1 induces dephosphorylation of Bcl-xL in a PP2A-dependent manner during oxidative stress and promotes retinal pigment epithelial cell survival. J. Biol. Chem. 2010;285:18301–18308. doi: 10.1074/jbc.M109.095232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldbaum O, Richter-Landsberg C. Activation of PP2A-like phosphatase and modulation of tau phosphorylation accompany stress-induced apoptosis in cultured oligodendrocytes. Glia. 2002;40:271–282. doi: 10.1002/glia.10119. [DOI] [PubMed] [Google Scholar]
- 40.Liu Q, Hofmann PA. Protein phosphatase 2A-mediated cross-talk between p38 MAPK and ERK in apoptosis of cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H2204–H2212. doi: 10.1152/ajpheart.01050.2003. [DOI] [PubMed] [Google Scholar]
- 41.Cheng WT, Guo ZX, Lin CA, Lin MY, Tung LC, Fang K. Oxidative stress promotes autophagic cell death in human neuroblastoma cells with ectopic transfer of mitochondrial PPP2R2B (Bβ2) BMC Cell Biol. 2009;10:91. doi: 10.1186/1471-2121-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magenta A, Fasanaro P, Romani S, Di Stefano V, Capogrossi MC, Martelli F. Protein phosphatase 2A subunit PR70 interacts with pRb and mediates its dephosphorylation. Mol. Cell. Biol. 2008;28:873–882. doi: 10.1128/MCB.00480-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janssens V, Jordens J, Stevens I, Van Hoof C, Martens E, De Smedt H, Engelborghs Y, Waelkens E, Goris J. Identification and functional analysis of two Ca2 + -binding EF-hand motifs in the B /PR72 subunit of protein phosphatase 2A. J. Biol. Chem. 2003;278:10697–10706. doi: 10.1074/jbc.M211717200. [DOI] [PubMed] [Google Scholar]
- 44.Ahn JH, Sung JY, McAvoy T, Nishi A, Janssens V, Goris J, Greengard P, Nairn AC. The B /PR72 subunit mediates Ca2 + -dependent dephosphorylation of DARPP-32 by protein phosphatase 2A. Proc. Natl. Acad. Sci. U.S.A. 2007;104:9876–9881. doi: 10.1073/pnas.0703589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cicchillitti L, Fasanaro P, Biglioli P, Capogrossi MC, Martelli F. Oxidative stress induces protein phosphatase 2A-dependent dephosphorylation of the pocket proteins pRb, p107, and p130. J. Biol. Chem. 2003;278:19509–19517. doi: 10.1074/jbc.M300511200. [DOI] [PubMed] [Google Scholar]
- 46.Kolupaeva V, Janssens V. PP1 and PP2A phosphatases: cooperating partners in modulating retinoblastoma protein activation. FEBS J. 2012 doi: 10.1111/j.1742-4658.2012.08511.x. doi:10.1111/j.1742-4658.2012.08511.x. [DOI] [PubMed] [Google Scholar]
- 47.Alvarez-Fernandez M, Halim VA, Aprelia M, Mohammed S, Medema RH. Protein phosphatase 2A (B55α) prevents premature activation of forkhead transcription factor FoxM1 by antagonizing cyclin A/cyclin-dependent kinase-mediated phosphorylation. J. Biol. Chem. 2011;286:33029–33036. doi: 10.1074/jbc.M111.253724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ackermann AM, Gannon M. Molecular regulation of pancreatic β-cell mass development, maintenance, and expansion. J. Mol. Endocrinol. 2007;38:193–206. doi: 10.1677/JME-06-0053. [DOI] [PubMed] [Google Scholar]