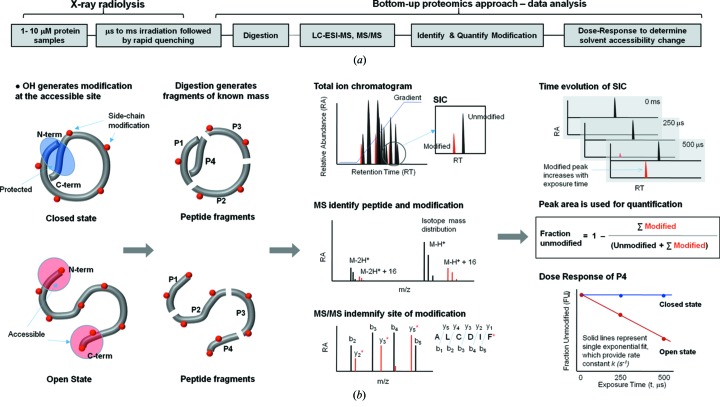
Figure 3.
Schematic of the X-ray footprinting method using synchrotron X-ray radiolysis and mass spectrometry. (a) Overall method, and (b) case study for a protein that undergoes a conformational change from the closed to the open state. The protein is covalently modified after a series of X-ray irradiations of the order of microseconds followed by rapid quenching by methionine amide. Irradiated protein is digested with proteases to generate peptide fragments of known mass. Digested protein is analyzed by reverse-phase liquid chromatography coupled with electrospray mass spectrometry (LC-ESI-MS), in which peptides are separated in the total ion chromatogram (TIC) or mass chromatogram. For any peptide fragment the unmodified and modified m/z is extracted and visualized by selected ion chromatogram (SIC). High-resolution mass spectrometry is used to identify the unmodified and modified peptide fragments by their accurate m/z and isotope distribution. The site of modification is identified from the mass assignment of the y and b fragment ions from the tandem mass spectrometry (MS/MS) of the corresponding peptide. The extent of modification for the series of irradiation points are quantified from the SICs of unmodified and modified pepide fragments. The fraction of unmodified peptide versus exposure time (dose-response or DR-plot) provides site-specific modification rate constants (k s−1). The rates are compared among different sample conditions, and their ratios, which are independent of intrinsic reactivity, account for the degree in solvent accessibility changes due to any conformational transition/interactions. The final results are mapped onto available structures or used as constraints for structural modeling.