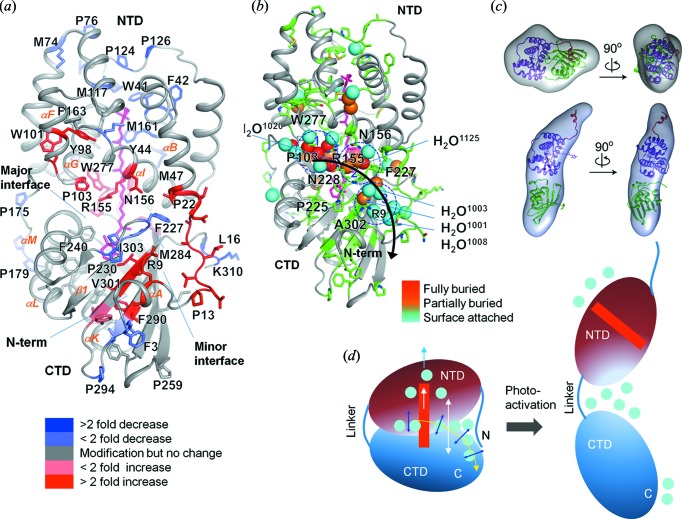
Figure 9.
XF-MS probes bound water mediated signal transfer pathway in OCP. (a) Solvent accessibility changes from dark adapted-OCPO to illuminated-OCPR are visualized on the structure of OCPO (3MG1). The modified residues are represented by sticks and the carotenoid is shown in pink. The color coding represents the ratio of rate constants between these two states. (c) The proposed signal propagation pathway from the carotenoid through the water side-chain hydrogen-bonding network to the protein surface that facilitates carotenoid shift, dissociation of NTD-CTD and detachment of the N-terminal helix from CTD. Conserved waters are shown in spheres and color codes indicate their depth from the surface of OCP. The modified residues are shown by green sticks. The results demonstrate disruption and reorganization of multiple close-packing interactions, mediated by both side chains and bound waters. (d) Ab initio bead reconstructions (gray volume) based on the SAXS results are shown for OCPO and OCPR. The subunit of OCPO from the crystal structure is docked into the volume envelope with the far N-terminal helix (red), NTD (purple) and CTD (green). The SAXS results show dissociation of NTD and CTD. (d) Schematic of the photoactivation of OCPO showing regions with the largest conformational rearrangement associated with changes in the hydrogen-bonding network and water rearrangements. Reproduced from Gupta et al. (2016 ▸).