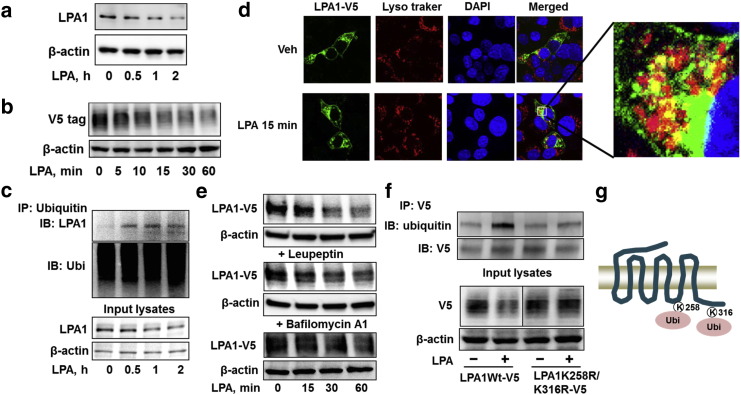
Fig. 1.
LPA1 lysosomal degradation is mediated by site specific ubiquitination. a. MLE12 cells were treated with LPA (5 μM) for 0–2 h, cell lysates were then analyzed by immunoblotting with LPA1 and β-actin antibodies. b. MLE12 cells were transfected with V5 tagged LPA1 (LPA1-V5) plasmid for 48 h, followed by LPA (5 μM, 0–60 min) treatment. Cell lysates were then analyzed by immunoblotting with V5 and β-actin antibodies. c. MLE12 cells were treated with LPA (5 μM) for 0–2 h. Cell lysates were then subjected to immunoprecipitation with an ubiquitin antibody, followed by LPA1 immunoblotting. Input lysates were analyzed by immunoblotting with LPA1 and β-actin antibodies. d. MLE12 cells grown on glass-bottom dishes were transfected with LPA1-V5 plasmid for 48 h, followed by LPA (5 μM, 15 min) treatment. Cells were fixed and stained with V5, lyso tracker, and 4′,6′-diamidino-2-phenylindole (DAPI). LPA1-V5, green; lysosome, red; nuclei, blue. e. MLE12 cells were transfected with Lpa1-V5 plasmid for 48 h, and then cells were treated with or without leupeptin (100 μM) or bafilomycin A1 (0.1 μM) for 1 h prior to LPA (5 μM, 0–60 min) treatment. Cell lysates were analyzed by immunoblotting with V5 and β-actin antibodies. f. MLE12 cells were transfected with Lpa1-V5 or Lpa1k258r&k316r-V5 plasmid for 48 h, and then cells were treated with LPA (5 μM, 1 h). Cell lysates were subjected to immunoprecipitation with a V5 antibody, followed by ubiquitin and V5 immunoblotting. Input lysates were analyzed by immunoblotting with V5 and β-actin antibodies. g. Scheme shows LPA1 is ubiquitinated on lysine 258 and 316 residues. Representative immunoblots were from at least three independent times.