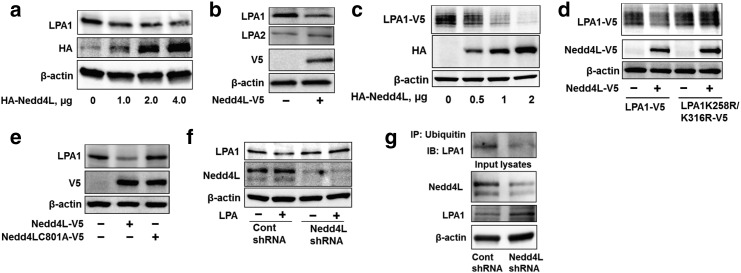
Fig. 2.
An E3 ubiquitin ligase, Nedd4L, induces LPA1 ubiquitination and degradation. a. MLE12 cells were transfected with HA-tagged Nedd4L (HA-NEDD4L, 0–4 μg) plasmid for 48 h. Cell lysates were analyzed by immunoblotting with LPA1, HA, and β-actin antibodies. b. HBEpCs were transfected with V5-tagged Nedd4L (NEDD4L-V5) plasmid for 48 h. Cell lysates were analyzed by immunoblotting with LPA1, LPA2, V5, and β-actin antibodies. c. MLE12 cells were co-transfected with LPA1-V5 and HA-NEDD4L (0–2 μg) plasmids for 48 h. Cell lysates were analyzed by immunoblotting with V5, HA, and β-actin antibodies. d. MLE12 cells were co-transfected with NEDD4L-V5 plasmid and LPA1-V5 or LPA1K258R&K316R-V5 plasmid for 48 h. Cell lysates were analyzed by immunoblotting with V5 and β-actin antibodies. e. MLE12 cells were transfected with NEDD4L-V5 or NEDD4LC801A-V5 plasmid for 48 h. Cell lysates were analyzed by immunoblotting with LPA1, V5, and β-actin antibodies. f. MLE12 cells were transfected with control shRNA (Cont shRNA) or Nedd4l shRNA for 72 h, and then cells were treated with LPA (5 μM) for 1 h. Cell lysates were analyzed by immunoblotting with LPA1, Nedd4L, and β-actin antibodies. g. MLE12 cells were transfected with Cont shRNA or Nedd4l shRNA for 72 h, and then cell lysates were subjected to immunoprecipitation with an ubiquitin antibody, followed by LPA1 immunoblotting. Cell lysates were analyzed by immunoblotting with LPA1, Nedd4L, and β-actin antibodies. Representative immunoblots were from at least three independent times.