Abstract
OBJECTIVE: Certain biomarkers such as the C-reactive protein, serum albumin, and the neutrophils to lymphocyte ratio are of prognostic significance regarding survival in different types of cancers. Data from sarcoma patients are sparse and mainly derived from soft tissue sarcoma and/or metastatic cases. Adjusting for confounders such as comorbidity and age is an essential safeguard against erroneous conclusions regarding the possible prognostic value of these biomarkers. The aim of this study was to assess the prognostic value of a battery of pretreatment biomarkers in the serum of patients with localized bone sarcomas and to adjust for potential confounders. MATERIAL AND METHODS: All patients diagnosed with localized intermediate and high-grade bone sarcoma during 1994 to 2008 were extracted from the Aarhus Sarcoma Registry. The serum levels of albumin, C-reactive protein, hemoglobin, neutrophils, lymphocytes, and sodium were collected from the patient records. The prognostic values of overall and disease-specific mortality were tested for each individual biomarker as well as for the Glasgow prognostic score (GPS) and for a new composite score incorporating five biomarkers (Aarhus composite biomarker score: ACBS). Adjustments were made for comorbidity as well as other possible prognostic factors, such as size, histological type, margin, chemotherapy, and soft tissue extension, using the Cox proportional hazard model. RESULTS: A total of 172 patients with high- or intermediate-grade localized bone sarcoma were included. Of these patients, 63 were diagnosed with chondrosarcoma and 109 patients with Ewing/osteosarcoma. The median age was 55 years for chondrosarcoma and 19 years for Ewing/osteosarcoma patients. The overall 5-year mortality was 31% [95% confidence interval (CI): 21-44] and 41% (95% CI: 33-51), whereas the 5-year disease-specific mortality was 21% (95% CI: 12-34) and 39% (95% CI: 31-49) for chondrosarcoma and Ewing/osteosarcoma, respectively. Comorbidities were present in 12% of the Ewing/osteosarcoma patients and in 24% of the chondrosarcoma patients. After adjustment for comorbidity and other confounders, it was found that elevated levels of CRP, low hemoglobin, low sodium, high GPS, and high ACBS were associated with increased overall mortality. Furthermore, elevated levels of CRP, low hemoglobin, high GPS, and high ACBS were associated with increased disease-specific mortality. CONCLUSION: Elevated levels of CRP, low hemoglobin, high GPS, and high ACBS were all independent prognostic factors for both overall and disease-specific mortality. ACBS is a new three-level score of five biomarkers, but its value has to be confirmed in an independent data set.
Introduction
The prognostic value of different serum biomarkers is well established in various cancers [1], [2], [3]. However, to avoid erroneous conclusions, adjustments for confounders such as comorbidity and age must be incorporated into such data analysis.
Very few studies have investigated the prognostic value of serum biomarkers in sarcoma patients [4], and therefore, little is known about their prognostic value, especially in bone sarcomas.
Bone sarcoma is a rare group of tumors dominated by osteosarcoma, Ewing sarcoma, and chondrosarcoma. Ewing sarcoma and osteosarcoma have similar epidemiological features, both with a peak incidence rate during the second decade of life [5], [6]. Osteosarcoma has a second incidence peak after 60 [7]. Chondrosarcoma has a gradual increase in incidence rate up to 75 years of age [5], [6]. Treatment failure is a major problem in clinical practice of bone sarcomas, and the 5-year survival rate for poor prognosis localized cases can be as low as 40% [5], [6], [7]. Although various prognostic factors are known, none of them could be used to guide treatment or change clinical outcome. The search for new and reliable prognostic factors that can help in allocating patients to the best treatment and improve the final outcome has to continue.
The aim of this study was to assess the prognostic value of serum biomarkers taken before the primary treatment of bone sarcoma adjusted for potential confounders.
A similar study is under preparation for soft tissue sarcomas, but the results will be reported in a separate study. This is because of the differences in age distribution, prognosis, treatment modalities, as well as histopathology between soft tissue and bone sarcomas.
Material and Methods
Study Cohort
All patients diagnosed with Ewing sarcoma, osteosarcoma, or chondrosarcoma and treated between January 1994 and December 2008 at Aarhus Sarcoma Centre, Denmark, were included in the present study. Patients with unclassifiable or low-grade tumors, metastasis at diagnosis, or no available blood samples were excluded from the analysis. This resulted in a cohort of 172 patients (Figure 1).
Figure 1.
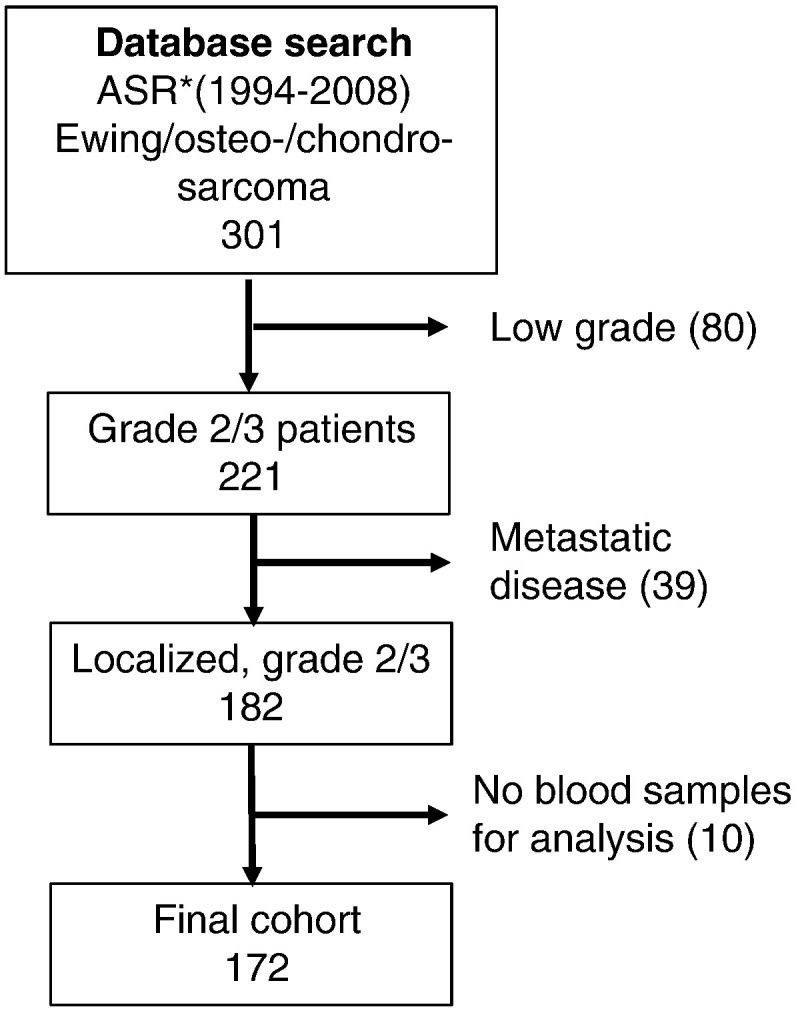
The number of patients included and excluded from the Aarhus Sarcoma Registry. The final study population comprises 171 patients.
Data Sources
Clinical data were obtained from the newly validated population-based Aarhus Sarcoma Registry [8], which contains comprehensive clinical information on each sarcoma patient from 1979 to 2008 in a well-defined geographic area of Denmark. Patients were diagnosed and treated, according to national guidelines, by an experienced multidisciplinary sarcoma team.
Biomarkers data were obtained from the clinical laboratory information system (LABKA) research database, which contains every blood test taken at any hospital in the northern and central regions of western Denmark since 2000 [9]. If it was not possible to obtain the biomarker results from the LABKA research database, the medical files were reviewed. The LABKA database registers test results according to the international nomenclature, properties, and units coding system [10].
The values selected for analysis included a time span from 30 days prior to sarcoma diagnosis to the day before the first treatment.
Serum albumin, C-reactive protein (CRP), hemoglobin, lymphocytes, neutrophils, and sodium were selected for analysis based on review of literature data. Each biomarker was categorized into normal or high/low according to the reference value in Aarhus University hospital at the time. Hypoalbuminemia was defined as albumin levels < 36 g/l or < 542 μmol/l. Elevated CRP was defined as values ≥ 8 mg/l or ≥ 75 nmol/l. Low hemoglobin was defined as levels < 7.3 mmol/l in females and < 8.3 mmol/l in males. Low sodium was defined as values < 137 mmol/l. Elevated neutrophils to lymphocytes ratio (NLR) was defined as > 5.3. Glasgow prognostic score (GPS) [1], [11] was defined as follows: normal, 1 if either the level of albumin was low or CRP was high, and 2 if both albumin level was low and the CRP level was high according to the reference levels stated above. A new biomarker score, the “Aarhus composite biomarker score” (ACBS) based on albumin, CRP, neutrophils, lymphocytes, and hemoglobin, was investigated. A score of 0 means that all serum biomarkers were within the normal range. A score of 1 means that only one biomarker was abnormal, and a score of 2 was obtained if more than one abnormal blood test was observed.
The National Patient Registry [12] was used to obtain data on comorbidities. All discharge diagnoses from 1 January 1977 until the date of sarcoma diagnosis were used. Diagnoses within 30 days and all cancer diagnoses within 90 days prior to the primary diagnosis of sarcoma were excluded.
Data Analysis and Statistics
Since 1968, all citizens in Denmark have been assigned a unique 10-digit civil personal registration number, which is used throughout all the Danish administrative registries and clinical databases. This allows for unambiguous linking and tracking of all patients. The data from Aarhus Sarcoma Registry, LABKA, and the National Patient Registry were therefore linked on an individual level using the civil personal registration number. The vital status and cause of death were registered through linkage to the Central Population Registry and Cause of Death Registry [13].
Patient-, tumor-, and treatment-related variables were reported according to each biomarker level and compared by using the chi-squared test. The primary end points were overall and disease-specific mortality. Death with sarcoma was regarded as a disease-specific event. The study period ended in 9 October 2013, and patients alive at this date were censored. The 5-year overall or disease-specific mortalities were reported by cumulative incidence functions for NLR, GPS, and ACBS using the Fine and Gray competing risk model [14]. Crude and adjusted analyses were performed by using the Cox proportional hazard model. The following variables were included in the adjusted analysis: age, comorbidity, size of the primary tumor, histological type, margin, grade, and soft tissue extension. Tumor size was included as a continuous variable; all others were analyzed as categorical variables as follows: age (≤ 40 vs > 40), comorbidity (yes versus no), histological type (Ewing/osteosarcoma versus chondrosarcoma vs. others), margin (wide versus nonwide), grade (grade 2 versus grade 3), and soft tissue extension (yes versus no).
To evaluate the value of the ACBS, we have tested the Cox proportional hazard model with the ACBS against the model without the ACBS using likelihood-ratio test.
As a way of comparison between the three different scores (ACBS, GPS, and NLR), we have used the Akaike information criterion (AIC).
The bootstrapping method with 1000 iterations was used as a form of validation of the ACBS score.
For all statistical tests, a two-sided P value less than .05 was regarded as significant. All statistical analyses were performed by using Stata version 14.
Ethics
The Ethics Committee of Denmark (1-10-72-233-12) and the Danish Agency of Data Protection (1-16-02-169-12) approved the study.
Results
Patients, Tumor, and Treatment Characteristics
A total of 172 patients with localized bone sarcoma were included in this analysis including 63 patients with chondrosarcoma and 109 with Ewing/osteosarcoma. The median age was 55 years for chondrosarcoma patients and 19 years for Ewing/osteosarcoma patients. Comorbidities were present in 12% of the Ewing/osteosarcoma patients and 24% of the chondrosarcoma patients.
The primary tumors were located in the lower extremities (n = 77), upper extremities (n = 30), trunk wall including pelvis (n = 48), and head (n = 17). Patient characteristics are shown in Table 1. The median follow-up was 8.8 years (range, 4.3 to 19 years) for patients alive at the end of follow-up. Patient characteristics according to the biomarkers are seen in Table 2.
Table 1.
Patients Characteristic. All Grade 2 and 3 Localized Bone Sarcoma Patient Divided Into Different Histological Tyypes (n=172)
| Total | Ewing/Osteosarcoma | Chondrosarcoma | P Value | |
|---|---|---|---|---|
| Number | 109 | 63 | ||
| Age (years) | ||||
| Median (range) | 28(2-83) | 19(2-75) | 55(16-83) | |
| Sex | ||||
| Female | 74(43) | 48(44) | 26(41) | |
| Male | 98(57) | 61(56) | 37(59) | .72 |
| Comorbidity | ||||
| No | 144(84) | 96(88) | 48(76) | |
| Mild | 12(7) | 4(4) | 8(13) | |
| Moderate/severe | 16(9) | 9(8) | 7(11) | .06 |
| Tumor size (cm)⁎ | ||||
| Median (range) | 9(2-30) | 9(2-21) | 9(3-30) | |
| Soft tissue extension | ||||
| No | 29(17) | 16(15) | 13(20) | |
| Yes | 143(83) | 97(85) | 50(79) | .32 |
| Malignancy grade | ||||
| 2 | 44(26) | 1(1) | 43(68) | |
| 3 | 128(74) | 108(95) | 20(32) | < .0001 |
| Treatment | ||||
| Surgery | 68(40) | 12(11) | 56(89) | |
| Surgery + Rt | 2(1) | 0 | 2(3) | |
| Surgery + Ch | 73(42) | 71(65) | 2(2) | |
| Surgery + Ch + Rt | 19(11) | 19(17) | 0 | |
| Ch | 2(1) | 2(2) | 0 | |
| Ch + Rt | 4(2) | 4(4) | 0 | |
| No treatment | 4(2) | 1(1) | 3(5) | < .0001 |
| Margin | ||||
| Wide/radical | 123(72) | 85(78) | 38(60) | |
| Intralesional/marginal | 39(23) | 17(16) | 22(35) | .014 |
| Recurrent disease | ||||
| No | 130(60) | 63(58) | 40(63) | |
| Yes | 69(40) | 46(42) | 23(37) | .46 |
| Local | 24(35) | 10(22) | 14(61) | |
| Lung | 23(33) | 20(43) | 3(13) | |
| Distant | 22(32) | 16(35) | 6(26) | .003 |
Abbreviations: Rt, radiotherapy; Ch, chemotherapy.
Eight missing values.
Table 2.
Patient Characteristics by Biomarkers (N = 172)
| Albumin Level (a) |
CRP Level (b) |
Hemoglobin Level (c) |
Neutrophil Level (d) |
Lymphocyte Level (e) |
Sodium Level (f) |
NLR (g) |
GPS (h) |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Normal | Low | P Value | Normal | High | P Value | Normal | Low | P Value | Normal | High | P Value | Normal | Low | P Value | Normal | Low | P Value | Normal | high | P Value | 0 | 1 | 2 | P Value | |
| Age (years) | ||||||||||||||||||||||||||
| 0-17 | 48(28) | 41(26) | 7(50) | 29(27) | 15(38) | 33(24) | 15(41) | 37(25) | 10(59) | 43(32) | 4(14) | 45(28) | 3(27) | 44(29) | 3(27) | 27(26) | 14(36) | 3(60) | ||||||||
| 18-40 | 57(33) | 54(34) | 3(21) | 35(33) | 11(27) | 48(36) | 9(24) | 48(33) | 5(29) | 42(31) | 11(38) | 54(34) | 3(37) | 47(31) | 6(55) | 35(34) | 9(23) | 2(40) | ||||||||
| 40 + | 67(39) | 63(40) | 4(29) | .16 | 42(40) | 14(35) | .49 | 54(40) | 13(35) | .14 | 61(42) | 2(12) | .01 | 49(37) | 14(48) | .14 | 62(39) | 5(45) | .88 | 61(40) | 2(18) | .22 | 40(39) | 16(41) | 0 | .23 |
| Sex | ||||||||||||||||||||||||||
| Female | 74(43) | 66(42) | 8(57) | 46(43) | 19(48) | 61(45) | 13(35) | 59(40) | 10(59) | 59(44) | 10(34) | 68(42) | 6(54) | 64(42) | 5(45) | 44(43) | 18(46) | 3(60) | ||||||||
| Male | 98(57) | 92(58) | 6(43) | .27 | 60(57) | 21(52) | .66 | 74(55) | 24(65) | .27 | 87(60) | 7(41) | .15 | 75(56) | 19(66) | .35 | 93(58) | 5(45) | .43 | 88(58) | 6(55) | .83 | 58(57) | 21(54) | 2(40) | .74 |
| Year of diagnosis | ||||||||||||||||||||||||||
| 1994-2000 | 63(36) | 57(36) | 6(42) | 31(29) | 11(28) | 47(35) | 16(43) | 54(37) | 5(29) | 47(35) | 12(41) | 61(38) | 2(18) | 56(37) | 3(27) | 29(28) | 12(31) | 1(20) | ||||||||
| 2001-2008 | 109(63) | 101(63) | 8(57) | .61 | 75(71) | 29(72) | .84 | 88(65) | 21(57) | .35 | 92(63) | 12(71) | .54 | 87(65) | 17(58) | .52 | 100(62) | 9(82) | .19 | 96(63) | 8(73) | .52 | 73(72) | 27(69) | 4(80) | .87 |
| Comorbidity | ||||||||||||||||||||||||||
| No | 144(83) | 133(84) | 11(79) | 90(85) | 34(85) | 117(87) | 27(73) | 123(84) | 15(88) | 118(88) | 20(69) | 136(84) | 8(73) | 131(86) | 7(64) | 87(85) | 32(82) | 5(100) | ||||||||
| Mild | 12(7) | 11(7) | 1(7) | 10(9) | 2(5) | 10(7) | 2(5) | 10(7) | 1(6) | 6(4) | 5(17) | 12(7) | 0 | 7(5) | 4(36) | 9(9) | 3(8) | 0 | ||||||||
| Moderate/severe | 16(9) | 14(9) | 2(14) | .8 | 6(6) | 4(10) | .47 | 8(6) | 8(22) | .01 | 13(9) | 1(6) | .9 | 10(7) | 4(14) | .02 | 13(8) | 3(27) | .08 | 14(9) | 0 | < .01 | 6(6) | 4(10) | 0 | .78 |
| Histological type | ||||||||||||||||||||||||||
| Ewing/Osteosarcoma | 109(63) | 98(62) | 11(79) | 63(59) | 27(68) | 83(61) | 26(70) | 91(62) | 15(88) | 91(68) | 15(52) | 103(64) | 6(55) | 98(64) | 8(73) | 60(59) | 25(64) | 5(100) | ||||||||
| Chondrosarcoma | 63(36) | 60(38) | 3(21) | .22 | 43(41) | 13(32) | .37 | 52(39) | 11(30) | .33 | 55(38) | 2(12) | .03 | 43(32) | 14(48) | .1 | 58(36) | 5(45) | .53 | 54(35) | 3(27) | .58 | 42(41) | 14(36) | 0 | .17 |
| Tumor size (cm)* | ||||||||||||||||||||||||||
| ≤ 5≤5 | 38(22) | 36(23) | 2(14) | 22(21) | 6(15) | 32(24) | 6(16) | 31(21) | 3(17) | 26(19) | 8(28) | 35(22) | 3(27) | 31(20) | 3(27) | 22(22) | 5(13) | 1(20) | ||||||||
| > 5 | 134(78) | 122(77) | 12(86) | .46 | 84(79) | 34(85) | .43 | 103(76) | 31(84) | .33 | 115(79) | 14(82) | .73 | 108(81) | 21(72) | .33 | 126(78) | 10(73) | .67 | 121(80) | 8(73) | .59 | 80(78) | 34(87) | 4(80) | .5 |
| Soft tissue involvement | ||||||||||||||||||||||||||
| No | 29(17) | 28(18) | 1(7) | 18(17) | 4(10) | 24(18) | 5(14) | 29(20) | 0 | 21(16) | 8(28) | 28(17) | 1(9) | 28(18) | 1(9) | 18(18) | 4(10) | 0 | ||||||||
| Yes | 143(83) | 130(82) | 13(93) | .31 | 88(83) | 36(90) | .29 | 111(82) | 32(86) | .54 | 117(80) | 17(100) | .04 | 113(84) | 21(72) | .13 | 133(82) | 10(92) | .48 | 124(82) | 10(91) | .44 | 84(82) | 35(90) | 5(100) | .35 |
| Malignancy grade | ||||||||||||||||||||||||||
| 2 | 44(26) | 41(26) | 3(21) | 34(32) | 5(13) | 39(28) | 5(14) | 38(26) | 1(6) | 31(23) | 8(28) | 41(25) | 3(27) | 37(24) | 2(18) | 33(32) | 6(15) | 0 | ||||||||
| 3 | 128(74) | 117(74) | 11(79) | .71 | 72(68) | 35(73) | .02 | 96(71) | 32(86) | .06 | 108(74) | 16(94) | .07 | 103(77) | 21(72) | .61 | 120(75) | 9(72) | .89 | 115(76) | 9(82) | .64 | 69(68) | 33(85) | 5(100) | .05 |
| Treatment | ||||||||||||||||||||||||||
| Surgery | 68(39) | 66(42) | 2(14) | 45(42) | 14(35) | 56(41) | 12(32) | 62(42) | 1(6) | 47(35) | 16(55) | 64(40) | 4(36) | 61(40) | 2(18) | 44(43) | 15(38) | 1(13) | ||||||||
| Surgery+Rt | 2(1) | 2(1) | 0(0) | 2(2) | 0 | 2(1) | 0 | 2(1) | 0 | 1(1) | 1(3) | 2(1) | 0 | 1(1) | 1(9) | 2(2) | 0 | 1(13) | ||||||||
| Surgery+Ch | 73(42) | 68(43) | 5(36) | 45(42) | 16(40) | 58(43) | 15(41) | 62(42) | 8(47) | 61(46) | 9(31) | 69(43) | 4(36) | 65(43) | 5(45) | 44(43) | 14(36) | 3(37) | ||||||||
| Surgery+Ch+Rt | 19(11) | 15(9) | 4(29) | 9(8) | 7(17) | 12(9) | 7(19) | 15(10) | 4(24) | 17(13) | 2(7) | 17(11) | 2(18) | 17(11) | 2(18) | 8(8) | 6(15) | 2(25) | ||||||||
| Ch | 2(1) | 2(1) | 0(0) | 0 | 2(5) | 0 | 2(5) | 0 | 2(12) | 2(1) | 0 | 2(1) | 0 | 2(1) | 0 | 0 | 2(5) | 1(13) | ||||||||
| Ch+Rt | 4(2) | 2(1) | 2(14) | 3(3) | 0 | 4(3) | 0 | 3(2) | 1(6) | 4(3) | 0 | 4(2) | 0 | 4(3) | 0 | 2(2) | 1(3) | 0 | ||||||||
| No treatment | 4(2) | 3(2) | 1(7) | .01 | 2(2) | 1(3) | .13 | 3(2) | 1(3) | .06 | 2(1) | 1(6) | < .01 | 2(1) | 1(3) | .27 | 3(2) | 1(9) | .74 | 2(1) | 1(9) | .08 | 2(2) | 1(3) | 0 | .24 |
| Margin | ||||||||||||||||||||||||||
| Wide/radical | 123(71) | 115(72) | 8(57) | 75(71) | 30(75) | 99(73) | 24(65) | 110(75) | 9(53) | 94(70) | 25(86) | 116(72) | 7(63) | 111(73) | 8(63) | 73(72) | 28(72) | 4(80) | ||||||||
| Intralesinal/marginal | 39(23) | 36(23) | 3(21) | .03 | 26(25) | 7(88) | .56 | 29(21) | 10(27) | .57 | 31(21) | 4(24) | < .01 | 32(24) | 3(10) | .21 | 36(22) | 3(27) | .81 | 33(21) | 2(18) | .85 | 25(25) | 7(18) | 1(20) | .57 |
Overall and Disease-Specific Mortality
At the end of the follow-up period, 76 patients had died (25 patients with chondrosarcoma and 51 patients with Ewing/osteosarcoma), yielding a 5-year overall mortality of 31% [95% confidence interval (CI): 21-44] and 41% (95% CI: 33-51) for chondrosarcoma and Ewing/osteosarcoma, respectively.
Of the patients who died in the chondrosarcoma group, 16 patients (64%) died from sarcoma and 9 (36%) died from other causes. Of the patients who died in the Ewing/osteosarcoma group, 46 patients (90%) died from sarcoma and 5 (10%) died from other causes. The 5-year disease-specific mortality was 21% (95% CI: 12-34) and 39% (95% CI: 31-49) for chondrosarcoma and Ewing/osteosarcoma, respectively.
Prognostic Value of Individual Biomarkers
Crude univariate analysis of individual biomarkers showed that CRP, serum sodium, and hemoglobin were significant prognostic factors for overall survival, whereas only CRP and hemoglobin were significant for disease-specific survival. Adjusting for other known prognostic factors and confounders such as age, size of the primary tumor, histological type, margin, soft tissue extension, as well as comorbidity did not change any of these results. The crude and adjusted results are illustrated in Table 3.
Table 3.
Crude and Adjusted Analysis (N = 172)
| Overall Mortality |
Disease-Specific Mortality |
||||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) |
HR (95% CI) |
||||||
| No. | Events | Crude | Adjusted | Events | Crude | Adjusted | |
| Albumin | |||||||
| Normal | 158 | 68 | 1 | 1 | 55 | 1 | 1 |
| Low | 14 | 8 | 1.9(0.9-3.9) | 2.8(0.9-4.8) | 7 | 2.1(1.0-4.6) | 1.7(0.7-4.1) |
| CRP | |||||||
| Normal | 106 | 32 | 1 | 1 | 27 | 1 | 1 |
| high | 40 | 29 | 3.6(2.2-6.0) | 3.6(2.1-6.3) | 24 | 3.5(2.0-6.1) | 3.6(2.0-6.5) |
| Missing | 26 | 15 | 11 | ||||
| Hemoglobin | |||||||
| Normal | 135 | 51 | 1 | 1 | 43 | 1 | 1 |
| Low | 37 | 25 | 2.3(1.4-3.8) | 1.9(1.1-3.1) | 19 | 2.2(1.3-3.7) | 1.8(1.0-3.2) |
| Sodium | |||||||
| Normal | 161 | 69 | 1 | 1 | 57 | 1 | 1 |
| Low | 11 | 7 | 2.3(1.0-5.0) | 2.6(1.2-5.8) | 5 | 2.0(0.8-4.9) | 2.1(0.8-5.2) |
| Lymphocytes | |||||||
| Normal | 134 | 56 | 1 | 1 | 46 | 1 | 1 |
| Low | 29 | 17 | 1.6(0.9-2.7) | 1.6(0.9-2.9) | 14 | 1.6(0.9-2.8) | 1.8(1.0-3.6) |
| Missing | 9 | 3 | 2 | ||||
| Neutrophils | |||||||
| Normal | 146 | 63 | 1 | 1 | 51 | 1 | 1 |
| High | 17 | 10 | 1.8(0.9-3.5) | 2.0(1.0-4.2) | 9 | 2.0(1.0-4.0) | 1.8(0.8-3.9) |
| Missing | 9 | 3 | 2 | ||||
| NLR | |||||||
| Normal | 152 | 66 | 1 | 1 | 54 | 1 | 1 |
| High | 11 | 7 | 2.0(0.9-4.4) | 2.2(1.0-5.2) | 6 | 2.1(0.9-5) | 2.3(0.9-5.5) |
| Missing | 9 | 3 | 2 | ||||
| GPS | |||||||
| Normal | 102 | 30 | 1 | 1 | 25 | 1 | 1 |
| 1 | 39 | 28 | 3.6(2.0-6.1) | 3.2(1.9-5.6) | 23 | 3.6(2.0-6.4) | 3.2(1.8-5.7) |
| 2 | 5 | 3 | 3.7(1.1-12) | 4.6(1.3-16) | 3 | 4.2(1.3-14) | 4.3(1.2-15) |
| Missing | 26 | 15 | 11 | ||||
| ACBS | |||||||
| Score = 0 | 73 | 18 | 1 | 1 | 15 | 1 | 1 |
| Score = 1 | 34 | 17 | 2.5(1.3-5) | 2.8(1.4-5.7) | 15 | 2.7(1.3-5.6) | 2.7(1.3-5.6) |
| Score = 2 | 36 | 26 | 4.4(2.4-8) | 3.6(1.9-6.9) | 21 | 4.2(2.2-8.2) | 3.6(1.8-7.2) |
| Missing | 29 | 15 | 11 | ||||
Adjustment were made for age, comorbidity, size, grade, histological type, margin, and soft tissue extension.
Prognostic Value of Composite Biomarkers
Crude analysis for the composite biomarkers (NLR, GPS, and ACBS) showed GPS and ACBS to be significant prognostic scores for both survival and disease-specific survival. Adjusting for the previously mentioned confounders including comorbidity did not affect these results. A significant difference was found between normal GPS and GPS = 1 (P = .001) but not between normal GPS and GPS = 2 (P = .061). Only five patients had GPS score of 2.
On the other hand, ACBS divided the patients into three prognostic groups with a reasonable number of patients in each group (See Table 4). There was a clear trend of increased 5-year overall mortality with increasing ASBC, from 15% (95% CI: 9-23) in patients with score of 0 to 47% (95% CI: 32-65) in patients with score of 1 and to 61% (95% CI: 46-77) in patients with score of 2.
Table 4.
The Distribution of Patients According to the GSP and ACBS Scores
| GPS | ||||
|---|---|---|---|---|
| ACBS | 0 | 1 | 2 | Total |
| 0 | 73 | 0 | 0 | 73 |
| 1 | 21 | 13 | 0 | 34 |
| 2 | 5 | 26 | 5 | 36 |
| Total | 99 | 39 | 5 | |
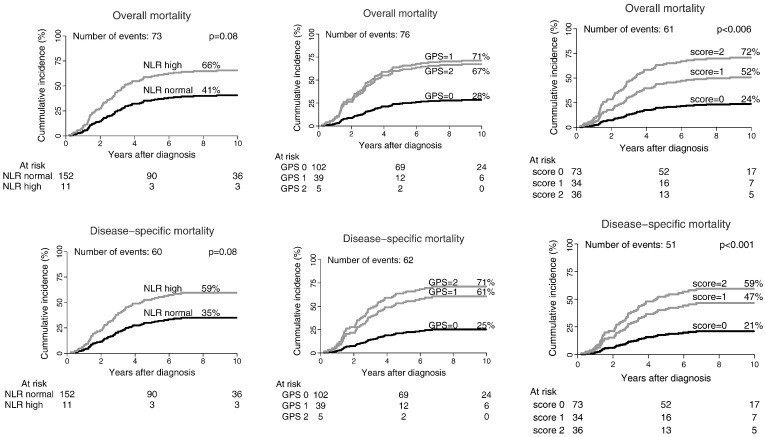
The adjusted cumulative overall mortality and disease-specific mortality for the various scores are shown in Figure 2. To estimate which of the three scores is best, the various prognostic scores (GPS, NLR, and ASBC) were compared using AIC. The least favorable prognostic score was the NLR with AIC = 568, whereas the GPS and ASBC had similar weights with AIC of 458 and 457, respectively.
Figure 2.
The cumulative incidence of overall and disease-specific mortality by GPS (n = 146), NLR (n = 163), and ACBS (n = 143). Biomarker score = 0: normal value for all investigated biomarkers. Biomarker score = 1: one abnormal marker. Biomarker score = 2: more than one abnormal biomarker. The analyses were performed using Fine and Gray competing risk model.
As validating the results in another data set is not currently feasible, we resorted to examining the ASBC using bootstrapping test with 1000 iterations. The test confirmed the value of the score as an independent prognostic factor with a hazard rate of 2.66 (95% CI: 1.08-6.45) for score of 1 and 3.59 (95% CI: 1.31-7.86) for score of 2.
Discussion
The use of various serum biomarkers in determining the prognosis for different types of cancer has been widely investigated [15], [16], [17], [18]. Most of these tested biomarkers are related to systemic inflammatory process of a sort. This is not surprising because it is now known that systemic inflammation can be associated with cancer development and progression [19]. The biochemical markers of inflammation include elevated CRP, hypoalbuminemia, and increased leukocytes and/or neutrophils. Many studies have pointed to the role of these various systemic inflammation-based prognostic biomarkers in different cancers. This also included the increase in the NLR [2], [20]. To refine the prognostic value of these serum biomarkers, different biomarker scores have been developed [3], [11], [20]. The most commonly used are the Glasgow score and NLR. However, these factors are unspecific. It is known that NLR values increase in acute pancreatitis [21], cardiac events [22], atherosclerosis, abnormal thyroid function, and old age. Moreover, different drugs such as angiotensin-converting enzyme inhibitors, angiotensin blockers, and statins [23] are able to affect the NLR. This underlines the importance of correcting for comorbidities.
Most studies on the role of biomarkers in the prognosis of sarcoma have been made for soft tissue sarcomas. In bone sarcomas, CRP, albumin level [24], [25], and lymphocyte and/or neutrophils [26], [27] were shown to be of prognostic value. These few studies, however, suffer from different problems such as not correcting for confounders and the lack of pretreatment values or hazard ratio levels.
In the present study, we have investigated albumin, CRP, hemoglobin, neutrophils, lymphocytes, and sodium separately. We also tested scores such as GPS and NLR that include more than one variable. In addition, we are presenting a new composite biomarker score named “ACBS” that combines and includes all the biomarkers tested in this study.
It was not possible to make an analysis of Ewing/osteosarcoma and chondrosarcoma separately because of the low number of events in some of the biomarkers tested; therefore, the adjusted analyses were only performed for the whole group of bone sarcomas. This may be considered as a weakness, and other studies testing the biomarker scores in a larger material of single histopathological subtype may be needed.
We decided to report both the overall mortality and the disease-specific mortality because 39 % of the chondrosarcoma patients died from other causes than sarcoma compared with Ewing/osteosarcoma where only 10% died from other causes than sarcoma. The ability to link our patients’ data on individual basis with other Danish registries including the cause of death registry is unique and makes disease-specific estimate robust.
CRP is the only single biomarker that according to the literature is prognostic for both soft tissue [28], [29] and bone sarcomas [25]. In the present study, we found CRP to be prognostic for overall and disease-specific mortality even when adjusted for confounders such as the presence of comorbidities and age.
Low levels of hemoglobin were observed in 22% of the patients in this study and were shown to be an independent prognostic factor for both disease-specific mortality and overall mortality as shown by Nakamura et al. [30].
Elevated NLR was recorded in only 11 patients in the present study, and severe comorbidity as a causative factor could be excluded. We were not able to retrieve information about medication at the time of diagnosis, but by adjusting for comorbidity, we believe that any bias would be minimized.
The GPS score has been shown to be prognostic for survival in different cancers including lung cancer [11], breast cancer [1], esophagus cancer [31], and kidney cancer [32]. This prognostic score has not yet been tested in bone sarcoma patients. We found that GPS was an independent prognostic factor for both overall mortality and disease-specific mortality when adjusted for different confounders. The score could not detect a difference in overall or disease-specific mortality between GPS = 1 and GPS = 2. This might be due to the low number of patients having a GPS value of 2 in this study.
Why ACBS and is it better than other scores?
The exact inflammatory process or mechanism behind the poor prognosis of a certain marker or score is not known. We felt therefore the need for another more comprehensive score that takes into account all the markers that were shown to be of prognostic value in our material. We have therefore tested a new biomarker score that equally weighs and categorizes the number of abnormal biomarker values taking CRP, albumin, neutrophils, lymphocytes, and hemoglobin into account (ABCS).
AIC is a measure for comparing maximum likelihood models. It was used here to compare the three composite scores (NLR, GPS, and ACBS). The model with the smaller value is considered to be better. In our analysis, the ACBS performed better than NLR but similar to GPS.
However, whereas GPS identified only 5 patients as belonging to the worst prognosis group, ACBS was able to identify 36 patients.
ACBS was thus able to separate the patients into three groups with reasonably equal number of patients in each group. ASBC was prognostic for both overall and disease-specific mortality. It also showed an obvious trend toward poorer prognosis with higher score. The difference between score 1 and 2 was not significant, which could be due to the low number of patients.
As we do not have access to a validation cohort, we decided to test the robustness of the ACBS score using bootstrapping test with 1000 iterations, and the results confirmed the value of the score as an independent prognostic factor in patients with localized bone sarcoma. Despite this confirmation, it is recommended that in order for ACBS to be incorporated into clinical practice, it has to be tested in a larger material preferably with one histopathological type.
In conclusion, this study showed that for patients with localized bone sarcomas, biomarkers such as elevated level of CRP and low hemoglobin and composite biomarkers scores such as GPS and ACBS are independent prognostic factors for both overall and disease-specific mortality. ACBS is a new three-level score of five biomarkers, but its value has to be confirmed in an independent data set.
Conflict of Interest
The authors declare that they have no competing interest
Acknowledgement
Authors’ contributions: N. A. P., K. M. N., J. K., S. B., and A. S.
Conception and design: all authors
Development of methodology: N. A. P., K. M. N.
Acquisition of data: all authors
Analysis and interpretation of data: N. A. P., K. M. N.
Writing the article: N. A. P.
Review and/or revision of the manuscript: all authors
Study supervision: J. K., S. B., and A. S.
Footnotes
Support: The study was supported by a scholarship from Aarhus University.
References
- 1.Al Murri AM, Bartlett JM, Canney PA, Doughty JC, Wilson C, McMillan DC. Evaluation of an inflammation-based prognostic score (GPS) in patients with metastatic breast cancer. Br J Cancer. 2006;94:227–230. doi: 10.1038/sj.bjc.6602922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams KA, Labidi-Galy SI, Terry KL, Vitonis AF, Welch WR, Goodman A, Cramer DW. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol. 2014;132:542–550. doi: 10.1016/j.ygyno.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer. 2014;134:2403–2413. doi: 10.1002/ijc.28536. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura T, Matsumine A, Matsubara T, Asanuma K, Uchida A, Sudo A. Clinical significance of pretreatment serum C-reactive protein level in soft tissue sarcoma. Cancer. 2012;118:1055–1061. doi: 10.1002/cncr.26353. [DOI] [PubMed] [Google Scholar]
- 5.Whelan J, McTiernan A, Cooper N, Wong YK, Francis M, Vernon S, Strauss SJ. Incidence and survival of malignant bone sarcomas in England 1979-2007. Int J Cancer. 2012;131:E508–E517. doi: 10.1002/ijc.26426. [DOI] [PubMed] [Google Scholar]
- 6.Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing's sarcoma: National Cancer Data Base report. Clin Orthop Relat Res. 2007;459:40–47. doi: 10.1097/BLO.0b013e318059b8c9. [DOI] [PubMed] [Google Scholar]
- 7.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maretty-Nielsen K, Aggerholm-Pedersen N, Keller J, Safwat A, Baerentzen S, Pedersen AB. Population-based Aarhus sarcoma registry: validity, completeness of registration, and incidence of bone and soft tissue sarcomas in western Denmark. Clin Epidemiol. 2013;5:45–56. doi: 10.2147/CLEP.S41835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grann AF, Erichsen R, Nielsen AG, Froslev T, Thomsen RW. Existing data sources for clinical epidemiology: the clinical laboratory information system (LABKA) research database at Aarhus University, Denmark. Clin Epidemiol. 2011;3:133–138. doi: 10.2147/CLEP.S17901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joint Committee on Nomenclature, Properties and Units (C-SC-NPU) of the IFCC and IUPAC, Pontet F, Magdal Petersen U, Fuentes-Arderiu X, Nordin G, Bruunshuus I, Ihalainen J, Karlsson D, Forsum U, Dybkaer R. Clinical laboratory sciences data transmission: the NPU coding system. Stud Health Technol Inform. 2009;150:265–269. [PMC free article] [PubMed] [Google Scholar]
- 11.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non–small-cell lung cancer. Br J Cancer. 2003;89:1028–1030. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39:30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 13.Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health. 2011;39:26–29. doi: 10.1177/1403494811399958. [DOI] [PubMed] [Google Scholar]
- 14.Fine Jand Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 15.Peter F, Wittekindt C, Finkensieper M, Kiehntopf M, Guntinas-Lichius O. Prognostic impact of pretherapeutic laboratory values in head and neck cancer patients. J Cancer Res Clin Oncol. 2013;139:171–178. doi: 10.1007/s00432-012-1320-1. [DOI] [PubMed] [Google Scholar]
- 16.Villasenor A, Flatt SW, Marinac C, Natarajan L, Pierce JP, Patterson RE. Postdiagnosis C-reactive protein and breast cancer survivorship: findings from the WHEL study. Cancer Epidemiol Biomarkers Prev. 2014;23:189–199. doi: 10.1158/1055-9965.EPI-13-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stenman M, Laurell A, Lindskog M. Prognostic significance of serum albumin in patients with metastatic renal cell carcinoma. Med Oncol. 2014;31:841. doi: 10.1007/s12032-014-0841-7. [014-0841-7. Epub 2014 Jan 30] [DOI] [PubMed] [Google Scholar]
- 18.Dai J, Tang K, Xiao W, Yu G, Zeng J, Li W, Zhang YQ, Xu H, Chen ZQ, Ye ZQ. Prognostic significance of C-reactive protein in urological cancers: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:3369–3375. doi: 10.7314/apjcp.2014.15.8.3369. [DOI] [PubMed] [Google Scholar]
- 19.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermanns T, Bhindi B, Wei Y, Yu J, Noon AP, Richard PO, Bhatt JR, Almatar A, Jewett MA, Fleshner NE. Pre-treatment neutrophil-to-lymphocyte ratio as predictor of adverse outcomes in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Br J Cancer. 2014;111:444–451. doi: 10.1038/bjc.2014.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azab B, Jaglall N, Atallah JP, Lamet A, Raja-Surya V, Farah B, Lesser M, Widmann WD. Neutrophil-lymphocyte ratio as a predictor of adverse outcomes of acute pancreatitis. Pancreatology. 2011;11:445–452. doi: 10.1159/000331494. [DOI] [PubMed] [Google Scholar]
- 22.Sawant AC, Adhikari P, Narra SR, Srivatsa SS, Mills PK, Srivatsa SS. Neutrophil to lymphocyte ratio predicts short and long term mortality following revascularization therapy for ST elevation myocardial infarction. Cardiol J. 2014;21:500–508. doi: 10.5603/CJ.a2013.0148. [DOI] [PubMed] [Google Scholar]
- 23.Karaman M, Balta S, Seyit Ahmet AY, Cakar M, Naharci I, Demirkol S, Celik T, Arslan Z, Kurt O, Kocak N. The comparative effects of valsartan and amlodipine on vWf levels and N/L ratio in patients with newly diagnosed hypertension. Clin Exp Hypertens. 2013;35:516–522. doi: 10.3109/10641963.2012.758734. [DOI] [PubMed] [Google Scholar]
- 24.Funovics PT, Edelhauser G, Funovics MA, Laux C, Berzaczy D, Kubista B, Kotz RI, Dominkus M. Pre-operative serum C-reactive protein as independent prognostic factor for survival but not infection in patients with high-grade osteosarcoma. Int Orthop. 2011;35:1529–1536. doi: 10.1007/s00264-011-1208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura T, Grimer RJ, Gaston CL, Watanuki M, Sudo A, Jeys L. The prognostic value of the serum level of C-reactive protein for the survival of patients with a primary sarcoma of bone. Bone Joint J. 2013;95-B:411–418. doi: 10.1302/0301-620X.95B3.30344. [DOI] [PubMed] [Google Scholar]
- 26.Aparicio J, Munarriz B, Pastor M, Vera FJ, Castel V, Aparisi F, Montalar J, Badal MD, Gomez-Codina J, Herranz C. Long-term follow-up and prognostic factors in Ewing's sarcoma. A multivariate analysis of 116 patients from a single institution. Oncology. 1998;55:20–26. doi: 10.1159/000011841. [DOI] [PubMed] [Google Scholar]
- 27.De Angulo G, Hernandez M, Morales-Arias J, Herzog CE, Anderson P, Wolff J, Kleinerman ES. Early lymphocyte recovery as a prognostic indicator for high-risk Ewing sarcoma. J Pediatr Hematol Oncol. 2007;29:48–52. doi: 10.1097/MPH.0b013e31802d3e3e. [DOI] [PubMed] [Google Scholar]
- 28.Szkandera J, Gerger A, Liegl-Atzwanger B, Absenger G, Stotz M, Samonigg H, Maurer-Ertl W, Stojakovic T, Ploner F, Leithner A. Validation of the prognostic relevance of plasma C-reactive protein levels in soft-tissue sarcoma patients. Br J Cancer. 2013;109:2316–2322. doi: 10.1038/bjc.2013.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi ES, Kim HS, Han I. Elevated preoperative systemic inflammatory markers predict poor outcome in localized soft tissue sarcoma. Ann Surg Oncol. 2014;21:778–785. doi: 10.1245/s10434-013-3418-3. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T, Grimer R, Gaston C, Carter S, Tillman R, Abudu A, Jeys L, Sudo A. The relationship between pretreatment anaemia and survival in patients with adult soft tissue sarcoma. J Orthop Sci. 2013;18:987–993. doi: 10.1007/s00776-013-0454-6. [DOI] [PubMed] [Google Scholar]
- 31.Crumley AB, McMillan DC, McKernan M, McDonald AC, Stuart RC. Evaluation of an inflammation-based prognostic score in patients with inoperable gastro-oesophageal cancer. Br J Cancer. 2006;94:637–641. doi: 10.1038/sj.bjc.6602998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tai CG, Johnson TV, Abbasi A, Herrell L, Harris WB, Kucuk O, Canter DJ, Ogan K, Pattaras JG, Nieh PT. External validation of the modified glasgow prognostic score for renal cancer. Indian J Urol. 2014;30:33–37. doi: 10.4103/0970-1591.124203. [DOI] [PMC free article] [PubMed] [Google Scholar]