Abstract
OBJECTIVES: Oxidative stress (OS) is an essential element in the pathogenesis of Barrett’s esophagus (BE) and its transformation to adenocarcinoma (EAC). The state of OS in the proximal stomach of patients with BE and EAC is unknown. Isoprostanes are a specific marker of OS not previously used to determine OS from BE/EAC tissue samples. PATIENTS AND METHODS: OS was measured in 42 patients with BE (n = 9), EAC (n = 9), or both (n = 24) and 15 control patients. A STAT-8-Isoprostane EIA Kit served to identify 8-Isoprostanes (8-IP), and a Glutathione Assay Kit was used to measure glutathione reduced form (GSH) and glutathione oxidized form. An OxiSelect Oxidative DNA Damage ELISA Kit (8-OHdG) served to measure 8-OH-deoxyguanosine. RESULTS: The 8-IP (P = .039) and 8-OHdG (P = .008) levels were higher, and the GSH level lower (P = .031), in the proximal stomach of the study group than in that of the controls. Helicobacter pylori infection was present in 8% of the study patients. CONCLUSIONS: In the proximal stomach of BE and EAC patients, OS was elevated and antioxidative capacity was reduced. This finding suggests that the gastroesophageal reflux causing BE also induces oxidative stress in the proximal stomach and may contribute to the development of cancer in the proximal stomach and gastric cardia.
Introduction
Adenocarcinoma of the esophagus and esophagogastric junction (EAC) is a disease with a poor prognosis and rising incidence. Its most important risk factors are gastroesophageal reflux disease (GERD) and Barrett’s esophagus (BE) [1]. Long-lasting GERD causes chronic inflammation and greater accumulation of intracellular reactive oxygen species (ROS), leading to a state of oxidative stress (OS) [2], [3], [4], [5]. ROS modify DNA bases, causing the production of DNA adducts that can serve as markers of DNA damage. These adducts can initiate mutagenic and carcinogenic processes by producing mispaired DNA sequences [2], [6], [7], [8], [9], [10]. ROS levels are known to be higher in tissues with BE and EAC [10], [11], [12], [13], [14], [15]. The most common marker of oxidative DNA damage is 8-OH-deoxyguanosine (8-OHdG), which plays an essential role in the induction of spontaneous mutations [16]. Its presence in BE, BE dysplasia, and EAC tissues is significantly higher than in the normal squamous esophageal epithelium [3].
The glutathione redox system and superoxide dismutase (SOD) function as a defense mechanism against OS [17], [18]. In previous studies, the GSH content and SOD contents were markedly lower in Barrett’s epithelium than in normal esophageal mucosa [12], [15], [19], [20].
8-Iso-PGF2 (8IP), a prostaglandin-like compound produced via cyclooxygenase-independent enzymes, is considered the most sensitive and reliable marker of lipid peroxidation and an indicator of oxidative status in vivo [21], [22]. Its circulating plasma levels have been associated with colon, prostate, and breast cancer [23], [24], [25], hence its association with malignant processes. To our knowledge, however, 8-Iso-PGF2 (8IP) has not been measured in BE or EAC tissue samples.
Inflammation of the gastric cardia is associated not only with Helicobacter pylori infection (HPI) due to pangastritis but also with erosive esophagitis and GERD in patients without HPI [26]. The association between cardiac inflammation and EAC is unclear, and most cases are associated with BE [27], [28], [29]. Because inflammation and OS are strongly linked, in this study, we evaluated whether OS is present and estimated the antioxidant capacity of the proximal stomach of patients with BE and EAC.
Materials and Methods
Patients
Our study included 57 patients (Table 1). Nine samples came from patients with only esophageal intestinal metaplasia (histologically observable goblet cells in the tubular esophagus), 9 from patients with EAC, and 24 with both. Eleven patients in EAC group had undergone neoadjuvant chemotherapy. The control group contained 15 patients with no reflux symptoms and a healthy esophagus in the endoscopic examination. The biopsies from the control patients also revealed healthy gastric mucosa. We evaluated the study patients’ records for a history of HPI. Patients in the control group were significantly younger (P = .015, independent-samples median test). The ethics committee of the Helsinki University Hospital approved the study protocol.
Table 1.
Patient Groups
| Group | n | Age (Year), Median (Range) |
|---|---|---|
| Control | 15 | 46 (20-75) |
| BE | 9 | 58 (33-81) |
| EAC | 9 | 69 (58-78) |
| BE and EAC | 24 | 63 (40-80) |
| Total | 57 |
Abbreviations: BE, Barrett´s esophagus; EAC, Esophageal adenocarcinoma.
Sample Collection
We acquired samples from follow-ups with Barrett’s patients and the pretreatment endoscopies of esophageal adenocarcinoma patients, or from resected specimens during surgery (11 of the EAC samples). In endoscopy, we sampled the most obvious area of pathology, including both the tumor and metaplastic mucosa, if present. Proximal gastric mucosal samples were taken 5 cm below the top of the proximal gastric folds. In the control patients, squamous epithelium samples were taken 5 cm above the gastroesophageal junction. All samples were immediately frozen and stored at − 70°C and later sent for analysis.
Methods
Before the biochemical analyses, we homogenized the tissue specimens in ice-cold 0.1 M Tris buffer with 1 mM EDTA and 10 mM indomethacin at pH 7.4. A STAT-8-Isoprostane EIA Kit (Cayman Chemical, Ann Arbor, MI) served to identify 8-Isoprostanes, and a Glutathione Assay Kit by Cayman Chemical was used to measure reduced glutathione (GSH) and oxidized glutathione forms (GSSG). An OxiSelect Oxidative DNA Damage ELISA Kit (8-OHdG) (Cell Biolabs, Inc., San Diego, CA) served to measure 8-OH-deoxyguanosine.
Statistical Methods
All statistical data are expressed as median (range). Mann-Whitney U test served to compare medians between patient groups. Significance was set to P < .05, and all P values were based on two-sided tests. SPSS software (SPSS Inc., Chicago, IL) served to calculate the statistics.
Results
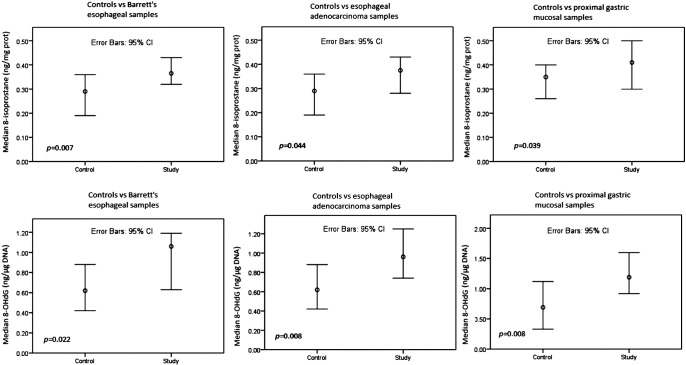
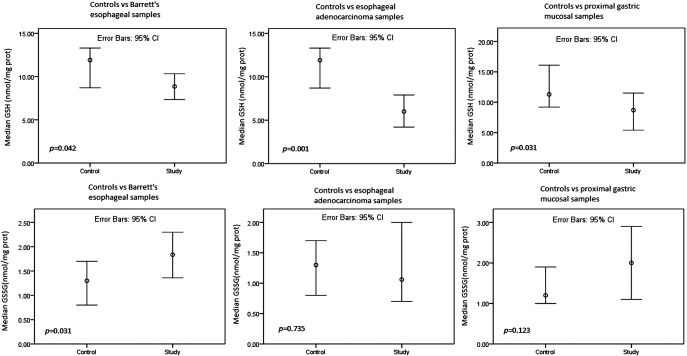
HPI was present in 3/38 (8%) of the study patients. The 8-IP (P = .039) and 8-OHdG (P = .008) content (Figure 1) was higher, and the GSH content (Figure 2) lower (P = .031), in the proximal stomach of study group patients than in that of the controls. In the BE samples, the 8-IP (P = .007) and 8-OHdG (P = .022) content (Figure 1) was higher and the GSH content (P = .042) (Figure 2) lower than in the control patients’ samples taken above the GE junction. In the EAC samples, the 8-IP (P = .044) and 8-OHdG (P = .008) content (Figure 1) was higher and the GSH content (P = .001) (Figure 2) lower than in the control patients’ samples taken above the GE junction. The study and control patients also showed no difference in GSSG levels (Figure 1).
Figure 1.
Comparisons of 8-IP and 8-OHdG levels between study and control groups. Mann-Whitney U test.
Figure 2.
Comparisons of GSH and GSSG levels between study and control groups. Mann-Whitney U test.
Discussion
Our study shows that active lipid peroxidation, as detected with elevated 8IP, was higher in the mucosa of the proximal stomach of BE and EAC patients than in that of healthy controls. Moreover, levels of 8-OHdG were elevated, indicating DNA damaged by ROS. GSH levels were also lower, suggesting either growing consumption or defective antioxidant capacity or both.
The etiology of adenocarcinoma in gastric cardia is considered more heterogenic than that of distal esophagus with intestinal metaplasia. Yamada et al. [28] evaluated 121 patients with cardiac EAC and found that 55% of all of the patients had histologic gastritis in the proximal stomach and 38% of patients with BE had gastritis. Etiology appeared to be multifactorial, with one third of cardiac EACs occurring without BE, likely from the proximal gastric mucosa. Of all of the patients, 85% also had esophagitis, which is a known risk factor even without BE [29]. Wijetunge et al. [30] reviewed gastric biopsies from 234 patients with either distal esophageal high-grade dysplasia or EAC, or cardiac EAC in comparison to controls with normal esophageal and gastric mucosa. Gastric biopsies were normal in 85.6% of study group patients, and the rest (14.4%) had either carditis, HPI, or gastric intestinal metaplasia. They found no differences related to pathological gastric mucosal findings between distal esophageal or cardiac EAC and concluded that most cardiac EACs have their origins in BE mucosa. This suggests that gastroesophageal reflux has a role in the inflammation and OS of the proximal stomach mucosa. Because all of our patients also had BE, most must also have GERD. Moreover, because the patients in our study group also had a low incidence of HPI, GERD being related to OS in the proximal stomach is a more likely explanation. Duodenogastric reflux (DGR) is known to cause gastritis in patients with partial gastrectomy [31]. It may be speculated that DGR is causing inflammation not only in the esophagus but also in gastric mucosa and thereby causing oxidative damage in cardiac region. This hypothesis is supported by results from Dixon et al. [32] pointing out association with DGR, inflammation, and intestinal metaplasia at cardia unrelated to HPI. Similar association was also suggested by Voutilainen et al. [26].
In a previous study by our group [12], DNA adducts were more numerous in the BE and EAC samples than in those of the control patients, and most numerous in the BE samples. GSH and DNA adduct levels also showed a negative correlation; this study shows the same results. In a study by Peters et al. [19], GSH levels were lower in BE and the gastric mucosa than in normal esophageal squamous epithelium or the duodenal mucosa, which is in line with our result. Low GSH levels in BE have shown an association with a risk for EAC, which also supports our results [33]. Another study by our group [14] showed that GSH and SOD levels in patients with GERD were lower than those of control patients in the proximal esophagus both before and after fundoplication, suggesting a primary deficiency in antioxidant capacity. This deficiency could also explain lower GSH levels in the proximal cardiac mucosa of our patients.
Our study shows that proximal gastric mucosa adjacent to metaplastic esophagus reacts to OS and links reflux of duodenal and gastric content in the pathogenesis of cardiac and distal EAC. Our study is limited by its small number of patients, and the control patients were younger than average. However, the data were collected prospectively, and this is the first report to use isoprostanes analyzed from tissue samples from BE and EAC patients.
Acknowledgement
The authors thank Yvonne Sundström for her skillful secretarial assistance.
Footnotes
Conflict of Interest: The authors state no conflicts of interest.
References
- 1.Spechler SJ, Souza RF. Barrett's esophagus. N Engl J Med. 2014;371:836-845. doi: 10.1056/NEJMra1314704. [DOI] [PubMed] [Google Scholar]
- 2.Kasai H, Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984;12:2137–2145. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasanen JV, Sihvo EI, Ahotupa MO, Farkkila MA, Salo JA. The expression of 8-hydroxydeoxyguanosine in oesophageal tissues and tumours. Eur J Surg Oncol. 2007;33:1164–1168. doi: 10.1016/j.ejso.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 5.Panayiotidis M. Reactive oxygen species (ROS) in multistage carcinogenesis. Cancer Lett. 2008;266:3–5. doi: 10.1016/j.canlet.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 6.Nestmann ER, Bryant DW, Carr CJ. Toxicological significance of DNA adducts: summary of discussions with an expert panel. Regul Toxicol Pharmacol. 1996;24:9–18. doi: 10.1006/rtph.1996.0059. [DOI] [PubMed] [Google Scholar]
- 7.Clemons NJ, McColl KE, Fitzgerald RC. Nitric oxide and acid induce double-strand DNA breaks in Barrett's esophagus carcinogenesis via distinct mechanisms. Gastroenterology. 2007;133:1198–1209. doi: 10.1053/j.gastro.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 8.Salminen JT, Ramo OJ, Ahotupa MO, Farkkila MA, Salo JA. Increased DNA adducts in Barrett's esophagus and reflux-related esophageal malignancies. Ann Med. 2002;34:565–570. doi: 10.1080/078538902321117779. [DOI] [PubMed] [Google Scholar]
- 9.Zhang HY, Hormi-Carver K, Zhang X, Spechler SJ, Souza RF. In benign Barrett's epithelial cells, acid exposure generates reactive oxygen species that cause DNA double-strand breaks. Cancer Res. 2009;69:9083–9089. doi: 10.1158/0008-5472.CAN-09-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dvorak K, Payne CM, Chavarria M, Ramsey L, Dvorakova B, Bernstein H, Holubec H, Sampliner RE, Guy N, Condon A. Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett's oesophagus. Gut. 2007;56:763–771. doi: 10.1136/gut.2006.103697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olyaee M, Sontag S, Salman W, Schnell T, Mobarhan S, Eiznhamer D, Keshavarzian A. Mucosal reactive oxygen species production in oesophagitis and Barrett's oesophagus. Gut. 1995;37:168–173. doi: 10.1136/gut.37.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sihvo EI, Salminen JT, Rantanen TK, Ramo OJ, Ahotupa M, Farkkila M, Auvinen MI, Salo JA. Oxidative stress has a role in malignant transformation in Barrett's oesophagus. Int J Cancer. 2002;102:551–555. doi: 10.1002/ijc.10755. [DOI] [PubMed] [Google Scholar]
- 13.Picardo SL, Maher SG, O'Sullivan JN, Reynolds JV. Barrett's to oesophageal cancer sequence: a model of inflammatory-driven upper gastrointestinal cancer. Dig Surg. 2012;29:251–260. doi: 10.1159/000341498. [DOI] [PubMed] [Google Scholar]
- 14.Rasanen JV, Sihvo EI, Rantanen TK, Ahotupa MO, Farkkila MA, Harjula A, Salo JA. Gastroesophageal reflux patients' defective antioxidative capacity in the proximal esophageal mucosa before antireflux surgery and also after 4-year follow-up. Ann Med. 2008;40:74–80. doi: 10.1080/07853890701668508. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez P, Piazuelo E, Sanchez MT, Ortego J, Soteras F, Lanas A. Free radicals and antioxidant systems in reflux esophagitis and Barrett's esophagus. World J Gastroenterol. 2005;11:2697–2703. doi: 10.3748/wjg.v11.i18.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussain SP, Harris CC. Molecular epidemiology of human cancer: contribution of mutation spectra studies of tumor suppressor genes. Cancer Res. 1998;58:4023–4037. [PubMed] [Google Scholar]
- 17.Ogino K, Oka S, Okazaki Y, Takemoto T. Gastric mucosal protection and superoxide dismutase. J Clin Gastroenterol. 1988;10(Suppl. 1):S129–S132. doi: 10.1097/00004836-198812001-00019. [DOI] [PubMed] [Google Scholar]
- 18.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 19.Peters WH, Roelofs HM, Hectors MP, Nagengast FM, Jansen JB. Glutathione and glutathione S-transferases in Barrett's epithelium. Br J Cancer. 1993;67:1413–1417. doi: 10.1038/bjc.1993.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wetscher GJ, Hinder RA, Bagchi D, Hinder PR, Bagchi M, Perdikis G, McGinn T. Reflux esophagitis in humans is mediated by oxygen-derived free radicals. Am J Surg. 1995;170:552–556. doi: 10.1016/s0002-9610(99)80014-2. [discussion 556–7] [DOI] [PubMed] [Google Scholar]
- 21.Basu S, Helmersson J. Factors regulating isoprostane formation in vivo. Antioxid Redox Signal. 2005;7:221–235. doi: 10.1089/ars.2005.7.221. [DOI] [PubMed] [Google Scholar]
- 22.Montuschi P, Barnes PJ, Roberts LJ., II Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 23.Kedzierska M, Olas B, Wachowicz B, Jeziorski A, Piekarski J. The lipid peroxidation in breast cancer patients. Gen Physiol Biophys. 2010;29:208–210. [PubMed] [Google Scholar]
- 24.Kong SY, Bostick RM, Flanders WD, McClellan WM, Thyagarajan B, Gross MD, Judd S, Goodman M. Oxidative balance score, colorectal adenoma, and markers of oxidative stress and inflammation. Cancer Epidemiol Biomarkers Prev. 2014;23:545–554. doi: 10.1158/1055-9965.EPI-13-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barocas DA, Motley S, Cookson MS, Chang SS, Penson DF, Dai Q, Milne G, Roberts LJ, 2nd, Morrow J, Concepcion RS. Oxidative stress measured by urine F2-isoprostane level is associated with prostate cancer. J Urol. 2011;185:2102–2107. doi: 10.1016/j.juro.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voutilainen M, Farkkila M, Mecklin JP, Juhola M, Sipponen P. Chronic inflammation at the gastroesophageal junction (carditis) appears to be a specific finding related to Helicobacter pylori infection and gastroesophageal reflux disease. The Central Finland Endoscopy Study Group. Am J Gastroenterol. 1999;94:3175–3180. doi: 10.1111/j.1572-0241.1999.01513.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Liu S, Zhang Y, Bi C, Xiao Y, Lin R, Huang B, Tian D, Ying S, Su M. Helicobacter pylori infection and gastric cardia cancer in Chaoshan region. Microbes Infect. 2014;16:840–844. doi: 10.1016/j.micinf.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Yamada M, Kushima R, Oda I, Mojtahed K, Nonaka S, Suzuki H, Yoshinaga S, Matsubara A, Taniguchi H, Sekine S. Different histological status of gastritis in superficial adenocarcinoma of the esophagogastric junction. Jpn J Clin Oncol. 2014;44:65–71. doi: 10.1093/jjco/hyt167. [DOI] [PubMed] [Google Scholar]
- 29.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 30.Wijetunge S, Ma Y, DeMeester S, Hagen J, DeMeester T, Chandrasoma P. Association of adenocarcinomas of the distal esophagus, "gastroesophageal junction," and "gastric cardia" with gastric pathology. Am J Surg Pathol. 2010;34:1521–1527. doi: 10.1097/PAS.0b013e3181eff133. [DOI] [PubMed] [Google Scholar]
- 31.Offerhaus GJ, van de Stadt J, Huibregtse K, Tersmette AC, Tytgat GN. The mucosa of the gastric remnant harboring malignancy. Histologic findings in the biopsy specimens of 504 asymptomatic patients 15 to 46 years after partial gastrectomy with emphasis on nonmalignant lesions. Cancer. 1989;64:698–703. doi: 10.1002/1097-0142(19890801)64:3<698::aid-cncr2820640322>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 32.Dixon MF, Mapstone NP, Neville PM, Moayyedi P, Axon AT. Bile reflux gastritis and intestinal metaplasia at the cardia. Gut. 2002;51:351–355. doi: 10.1136/gut.51.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brabender J, Lord RV, Wickramasinghe K, Metzger R, Schneider PM, Park JM, Holscher AH, DeMeester TR, Danenberg KD, Danenberg PV. Glutathione S-transferase-pi expression is downregulated in patients with Barrett's esophagus and esophageal adenocarcinoma. J Gastrointest Surg. 2002;6:359–367. doi: 10.1016/s1091-255x(02)00003-3. [DOI] [PubMed] [Google Scholar]