ABSTRACT
In theory, the immunotherapy of cancer should induce the selective destruction of cancer cells and a long-term specific protection, based on the specificity and memory of immunity. This contrasts with the collateral damages of conventional therapies and their toxic effects on host tissues. However, recent data suggest that chemotherapy may potentiate ongoing immune responses, through homeostatic mechanisms. Massive tumor death, empty “immune” niches and selected cytokines may act as a danger signal, alerting the immune system and amplifying pre-existing antitumor reactivity.
KEYWORDS: Chemotherapy, drug efflux, homeostasis, immunotherapy, IL-7/IL-15, lymphopenia, memory T cells
Abbreviations
- ABCB1
ATP-binding cassette sub-family B member 1
- ALL
acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- CAR
chimeric antigen receptors
- CMV
cytomegalovirus
- DC
dendritic cell
- EBV
Epstein Barr virus
- GVHD
graft-versus-host disease
- HIV
human immunodeficiency virus
- HMGB1
high mobility group box 1
- RAG
recombination activating gene
- SCID
severe combined immunodeficiency
- Treg
regulatory T lymphocyte
Introduction
Numerous observations in the past years in cancer patients and in rodents have suggested a counterintuitive effect of chemotherapy on the immune system, i.e. its capacity to potentiate the tumor-specific immune response. This synergy between chemotherapy and immunity is of major interest, as it may open the way to innovative combined therapies.
Several molecular mechanisms have been identified by which chemotherapy may result in increased immunity to tumor cells: (i) induction of immunogenic tumor cell death resulting in the release of danger signals, such as high mobility group box 1 (HMGB1), and effective presentation by dendritic cells (DCs); (ii) expression of formyl peptide receptor 1 on DCs, thereby favoring stable interactions with annexin-1-expressing dying cancer cells, DC maturation and cross-presentation of tumor-associated antigens;1 (iii) disruption of homeostasis and/or inhibition of the function of regulatory T lymphocytes (Tregs).
However, the effect of the chemotherapeutic treatment on T cells themselves remains elusive. Two factors may directly affect T cell survival and/or activation: their capacity to extrude drugs through ATP-binding cassette transporters and their ability to proliferate in a lymphopenic environment created by conventional cytotoxic treatments (Fig. 1).
Figure 1.
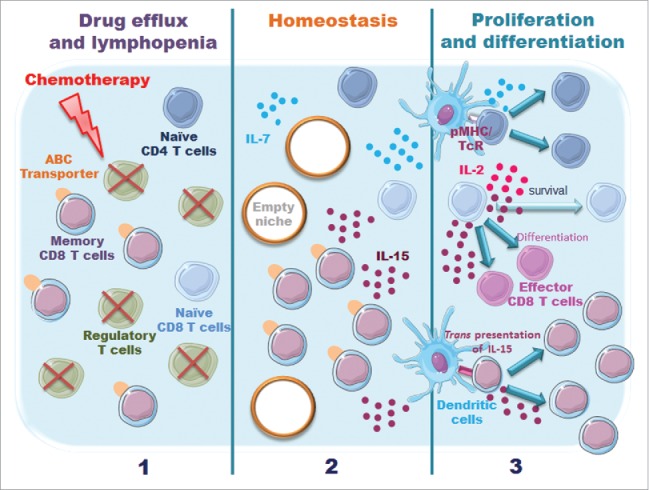
Model illustrating potential effects of chemotherapy on immune resistance. Two factors may affect T cell survival/function (i) their capacity to efflux drug via ATP-binding cassette (ABC) transporters; (ii) the availability of specific niches and their capacity to respond to homeostatic cytokines, such as IL-2, IL-7 and IL-15.
In this review, we will summarize experimental observations suggesting that the repertoire of reconstituted T cell memory after chemotherapy may be shaped by the drug efflux capacity and the stem cell like properties of tumor-specific T lymphocytes.
Memory and chemotherapy
Early observations suggested that memory T cells may resist chemotherapy. Indeed, a report compared infections between different treatments of children with acute myeloid leukemia (AML) and revealed similar fungal and viral (but increased bacterial) infections in patients treated with chemotherapy as compared to allogeneic stem cell transplantation.2,3 To test whether T cells were resistant and by which mechanism, the group of Riddell examined whether CD8+ T cells specific for viruses could be detected in the blood from adults after recovery from chemotherapy. These patients were treated with ATP-binding cassette sub-family B member 1 (ABCB1) chemotherapeutic substrates and developed profound lymphocytopenia (absolute blood counts < 100 cells/mL). After recovery (about 1000 cells/mL), PBMCs were stimulated in vitro with a pool of antigenic peptides from cytomegalovirus (CMV), Epstein Barr virus (EBV) and influenza viruses for 8 d with IL-2, IL-7 and IL-15 and the percentage of IFNγ-producing CD8+ T cells was estimated. Approximately 12% CD8+ T cells were detected, suggesting that a defined subset of memory T cells preferentially survived to the treatment. The authors identified subsets of central and effector memory cells which displayed high multidrug efflux capacity mediated by ABCB1 transporters, hence survived cytotoxic therapy in vivo, proliferated and were enriched during lymphopenia. These subsets are quiescent cells which may respond to antigens only in inflammatory conditions and display stem cells-like properties. These observations suggest that chemotherapy may potentiate immune resistance by favoring survival of selected clones from the spontaneous tumor-specific immune response.4
It is noteworthy that the increased immune resistance after chemotherapy may also result from decreased immunosuppression. Indeed, human regulatory T cells have been shown to lack the cyclophosphamide extruding transporter.5 As compared to conventional CD4+ T cells, this regulatory subset had a reduced capacity to efflux the fluorescent substrate (mitotracker green), did not express ABCB1 mRNA or protein and was more susceptible to cyclophosphamide-induced apoptosis. The lack of capacity to pump out cyclophosphamide may contribute to the selective susceptibility of this subset to this agent, together with the low ATP levels and impaired DNA repair mechanisms.
Surprisingly, very few observations were reported regarding the drug efflux capacity of T lymphocytes in humans, and even less in murine models.
Multiple questions remain to be answered and in particular the nature of the factor(s) that induce(s) the expression of the transporters and the link between stem-cell like properties and efflux capacity.
Memory and lymphopenia
One of the hallmarks of memory CD8+ T cells is their capacity of homeostatic proliferation. The analysis of persisting immune memory in yellow fever vaccines identified a subset of naive-like yellow fever-specific CD8+ T cells which persisted for decades and resembled stem-cell like memory cells (phenotypical similarity to naive cells and high stemness). The long-term persisting human memory CD8+ T cells from the vaccinees (but not unvaccinated individuals) efficiently responded to cognate peptides and IL-15-driven homeostatic proliferation, in contrast to the reference naive subset.6
As these lymphocytes share phenotypic features with cells expressing ABCB1 transporters, namely CD161 and IL-18R, it is tempting to speculate that the combination of efflux capacity and homeostatic proliferation to lymphopenic cytokines may provide a proliferative advantage to selected tumor-specific CD8+ T lymphocytes after chemotherapy, thus conferring long-term therapeutic efficacy.
Several factors may contribute to the activation of T cells in lymphopenic conditions:
Old studies have shown that the overall size of the T cell pool remains relatively constant and that T lymphocytes proliferate when niches are “empty.” In 2002, Ge et al. transferred lymph node cells from 2C TCR transgenic mice (consisting of >95 % naive T cells) into non-irradiated recombination activating gene-1−/− (RAG−/−) recipients or sublethally irradiated C57BL/6 hosts and examined their phenotype. They found that thymopoiesis and homeostasis-driven proliferation contributed to the reconstitution of naive and memory T cell compartments, respectively.7
The homeostatic proliferation mechanisms that control naive and memory T cells in lymphopenic conditions appear distinct. Proliferation of naive T cells has been shown to require TcR engagement by self-pMHC and IL-7 signaling. Indeed, naive T cells transferred into irradiated IL-7−/− mice failed to proliferate, and disappeared within a month, and the infusion of exogenous IL-7 restored their homeostatic proliferation.8 IL-7 signaling induces the expression of the anti-apoptotic genes, Bcl-2 and Mcl-1 and inhibits the pro-apoptotic genes, Bax and Bak, therefore regulating T cell survival.9-11 However, optimal survival of naive T cells, mainly for CD8+ T cells, requires other γc cytokines, such as IL-2 and IL-15 (for review, see12). By contrast, the survival and homeostatic proliferation of memory T cells are less dependent on TcR engagement, as CD4+13 and CD8+14 T cells were long-lived in the absence of MHC and retained their capacity to make rapid cytokine responses. The cytokine IL-15 appears critical for CD8+ T cell proliferation,15-18 whereas CD4+ T cells may require IL-7 and/or TcR-derived signals19 (for review, see20.). Of note, DCs express IL-15Rα, the high affinity receptor for IL-15, allowing trans-presentation and persistent IL-15 signaling.21 Cross-presentation by DCs may therefore not only induce TcR signaling but also regulate memory CD8+ T cells homeostasis.22
Different cytokines may be involved in slow vs. rapid homeostatic proliferation. Naive T cells transferred into γc-deficient hosts (thus unable to respond to IL-2, −4, −7, −9, −15 and −21) displayed fast proliferation and capacity to differentiate into central memory cells, probably due to increased cytokine availability. The proliferation was driven mainly by IL-15, with a lesser contribution from IL-2.23 It is noteworthy that the fast donor T cell proliferation was not observed if the hosts were raised under germfree condition, highlighting a role for the host microflora.24
Similarly in patients with T cell depletion of varying etiologies, evidence was found that increased availability of IL-7 could favor T cell homeostasis following T-cell depletion.25 Administration of IL-7 in patients with idiopathic CD4+ lymphopenia, a rare syndrome defined by low CD4+ T-cell counts (<300 cells /µL), led to an increase in the number of CD4+ and CD8+ T cells in peripheral blood and tissues, confirming the critical role of IL-7 for thymopoiesis, T cell homeostasis and/or survival.26
Lymphopenia and antitumor immunity
A few reports suggest that homeostatic expansion, in particular in a lymphopenic environment (induced by chemotherapy or irradiation), may improve the persistence and antitumoral function of T lymphocytes. A recent study by Ray-Coquard et al. revealed that lymphopenia may represent a valuable and simple prognostic factor for overall survival in three tumor types: metastatic breast carcinomas, advanced sarcoma and non-Hodgkin's lymphomas.27 Non-myeloablative lymphodepleting chemotherapy may also improve the efficacy of adoptive transfer therapy. Six of thirteen melanoma patients, who received immunodepleting chemotherapy (cyclophosphamide and fludarabine for 7 d) before adoptive transfer of tumor reactive T cells and IL-2, had objective clinical responses and four others displayed partial responses.28 These results were extended on a total of 35 patients with refractory metastatic melanoma.29
Similarly, growth of B78D14 melanoma cells was decreased in mice after sublethal irradiation and this effect was amplified by adoptive transfer of syngeneic T cells. This antitumor effect was mediated by polyclonal homeostatic T cell expansion, was independent from CD4+ T cells and correlated with elevated IFNγ production and B78D14-specific cytotoxicity.30
Several studies highlighted the role of IL-7 in lymphopenia-associated proliferation. IL-7 levels were shown to be increased in human immunodeficiency virus (HIV) patients and in patients after chemotherapy 25 (for review, see31). IL-7 displays pleiotropic actions on T cell immunity, increasing homeostatic expansion of CD4+ and CD8+ lymphocytes,32,33 extending the TcR repertoire diversity34 and stimulating thymus-dependent T-cell generation.35 Murine studies revealed that blockade of IL-7 signaling during lymphopenia-induced proliferation (using anti-IL-7R mAb) prevented the induction of antitumor activity, but had not effect at a later stage, suggesting that IL-7 is required for cell expansion rather than for expression of effector function.36
There is evidence that IL-15 may favor the differentiation of effector cells that persist for prolonged time and are highly effective in vivo. Indeed, tumor-specific CD8+ T cells cultured in the presence of IL-15 displayed a central memory phenotype (with low effector functions) but caused a strong reduction in the number of pulmonary metastases when transferred in tumor-bearing mice. By contrast, the same cells cultured in the presence of IL-2 exhibited potent effector function in vitro but failed to reduce the number of lung metastases.37 As trans-presentation of IL-15 by IL-15Rα is thought to be the dominant and most efficient mechanism of action of this cytokine, Steel et al. examined the antitumor effect of DCs engineered to express IL-15, IL-15Rα and NEU oncoprotein. Female BALB-neu T mice, transgenic for a transforming rat neu oncogene under the control of a chimeric mouse mammary tumor virus promoter, developed mammary tumors and died by about 20 weeks. Vaccination of mice with DCs expressing the triple combination resulted in survival of 70% of mice after 30 weeks. The antitumor effect was antibody dependent and independent of CD4+-help.38 Kaiser et al. further demonstrated that the memory-like CD8+ T cells (generated in vivo in a lymphopenic environment or in vitro using and-CD3/CD28 + IL-15 stimulation) displayed intrinsic antitumor potential, as they maintained their capacity to control tumor growth upon transfer in a non-lymphopenic host.39 Collectively, these observations suggest that homeostatic proliferation may be critical to generate T cells with high antitumor function.
CAR T cells
Very recently, a novel strategy has been developed which consists in the generation of T cells engineered to express chimeric antigen receptors (CAR) to specifically retarget tumor cells. This approach, that combines the specificity of an antibody with the cytotoxicity and memory functions of a T cell, has given impressive clinical results.40 The transfer of CAR T cells that specifically target the CD19 antigen has been shown to very efficiently control the growth of B cell malignancies in mice and humans. Complete response rates in approximately 90% of patients have been reported in adults and children with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) after adoptive transfer of CD19 CAR T cells expressing costimulatory domain CD28 or 4-1BB (for review, see41). Of note, human CAR T cells engineered to express the homeostatic cytokine IL-15 displayed superior survival and expansion in vitro and enhanced antitumor activity in vivo in severe combined immunodeficiency (SCID) mice.42
Regulatory T cells after lymphopenia
As early as in 1982, North showed that cyclophosphamide facilitated adoptive immunotherapy. Indeed, complete tumor regression was caused by infusion of cyclophosphamide and 1 h later tumor-immune spleen cells. However, tumor regression caused by the combination therapy was completely inhibited by intravenous infusion of cyclophosphamide-sensitive “suppressor” T cells from the spleens of tumor-bearing donors.43 Accordingly, as mentioned earlier, chemotherapeutic treatment, and in particular cyclophosphamide, may affect the survival of regulatory T cells. In addition, a few reports have examined the effect of lymphopenia itself on Treg homeostasis. Murine regulatory CD4+ T cells proliferated more strongly than the conventional CD4+ T cells upon transfer in CD3ϵ−/− host but their expansion was primarily controlled by IL-2 rather than IL-7.44 Co-transfer of conventional and regulatory T cells increased the extent of Treg expansion, pointing to conventional T cells as the source of IL-2. The role of Tregs was further highlighted by their capacity to prevent the rapid oligoclonal proliferation of conventional CD4+ T cells while preserving the slow homeostatic expansion, which favored protection to graft-versus-host disease (GVHD) in a bone marrow transplant model.45 Finally, mice lacking IL-7 had no sign of autoimmune disease and had a robust peripheral pool of Foxp3+ T cells that control inflammatory bowel disease.46 In humans, IL-7 administration led to expansion of CD4+ and CD8+ cells and broadening of the T cell repertoire, along with a decrease in the percentage of CD4+ regulatory T cells.33,34
Collectively, these data suggest that lymphopenia may enhance immunity through direct (by enhancing T cell effector function) and indirect (by inhibiting Treg function) mechanisms.
Conclusion
The dark side of conventional treatments to cancer, i.e., the toxic effect on most tissues, may in fact be of benefit to the host, by potentiating the ongoing tumor-specific immune response. The selected resistance to chemotherapeutic agents and/or the increased availability of homeostatic cytokines in lymphopenic conditions may indeed select T cells displaying strong cytotoxic and memory functions and restrict the repertoire of tumor-specific T cells to a defined subset of pre-activated effector/memory cells. Interestingly, homeostatic T cell proliferation has been shown to depend on gut microflora,24 an observation in line with two recent reports demonstrating that antibiotics may ablate the beneficial effect of chemotherapy.47,48
A key question is whether the beneficial effect of chemotherapy on the tumor itself and on the immune response may be uncoupled from the toxic effect on neighboring tissues. The mode of administration and the dose of cyclophosphamide may be determinant, as discussed in a recent review (for review, see49). In 2001, Machiels et al. concluded “that the optimal immune-modulating dose for three chemotherapeutic agents was just above the doses that began to cause cytopenia”,50 suggesting that a short chemotherapeutic treatment may be sufficient to boost the antitumor response.
Collectively, these observations are in favor of novel therapies that would combine immunotherapy with chemo/radiotherapy in order to exploit the potentiating effect of lymphopenia on tumor-specific immune resistance.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Oberdan Leo and Coralie Henin for interesting discussions.
Funding
The Laboratory of Immunobiology is supported by grants of the Fonds National de la Recherche Scientifique and by the Fondation contre le Cancer. A.H. is supported by the Fondation Rose et Jean Hoguet.
References
- 1.Erika V, Yuting M, Elisa E, Baracco AS, David PE, Federico P, Heng Y, Sandy A, Kariman C, Michaela S et al.. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science (80-)[Internet] 2015; 350:972-8. Available from: http://www.sciencemag.org/content/early/2015/10/28/science.aad0779.full.pdf; PMID:26516201; http://dx.doi.org/15920008 10.1126/science.aad0779 [DOI] [PubMed] [Google Scholar]
- 2.Haining WN, Neuberg DS, Keczkemethy HL, Evans JW, Rivoli S, Gelman R, Rosenblatt HM, Shearer WT, Guenaga J, Douek DC et al.. Antigen-specific T-cell memory is preserved in children treated for acute lymphoblastic leukemia. Blood [Internet] 2005; 106:1749-54. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1895221/; PMID:15920008; http://dx.doi.org/ 10.1182/blood-2005-03-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung L, Garnis A, Alonzo TA, Buxton A, Britton K, DeSwarte-Wallace J, Woods WG. Infections and association with different intensity of chemotherapy in children with acute myeloid leukemia. Cancer 2009; 115:1100-8; PMID:19156894; http://dx.doi.org/ 10.1002/cncr.24107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turtle CJ, Swanson HM, Fujii N, Estey EH, Riddell SR. A distinct subset of self-renewing human memory CD8+ T cells survives cytotoxic chemotherapy. Immunity 2009; 31:834-44; PMID:19879163; http://dx.doi.org/ 10.1016/j.immuni.2009.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimeloe S, Frick C, Fischer M, Gubser PM, Razik L, Bantug GR, Ravon M, Langenkamp A, Hess C. Human regulatory T cells lack the cyclophosphamideextruding transporter ABCB1 and are more susceptible to cyclophosphamide-induced apoptosis. Eur J Immunol 2014; 44:3614-20; PMID:25251877; http://dx.doi.org/ 10.1002/eji.201444879 [DOI] [PubMed] [Google Scholar]
- 6.Fuertes Marraco SA, Soneson C, Cagnon L, Gannon PO, Allard M, Maillard SA, Montandon N, Rufer N, Waldvogel S, Delorenzi M et al.. Long-lasting stem cell-like memory CD8+ T cells with a naive-like profile upon yellow fever vaccination. Sci Transl Med [Internet] 2015; 7:282ra48-282ra48. Available from http://stm.sciencemag.org/content/7/282/282ra48.abstract; PMID:25855494; http://dx.doi.org/ 10.1126/scitranslmed.aaa3700 [DOI] [PubMed] [Google Scholar]
- 7.Ge Q, Hu H, Eisen HN, Chen J. Different contributions of thymopoiesis and homeostasis-driven proliferation to the reconstitution of naive and memory T cell compartments. PNAS 2002; 99:2989-94; PMID:11880642; http://dx.doi.org/ 10.1073/pnas.052714099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. PNAS 2001; 98:8732-7; PMID:11447288; http://dx.doi.org/ 10.1073/pnas.161126098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim K, Lee C, Sayers TJ, Muegge K, Durum SK. The trophic action of IL-7 on pro-T cells: inhibition of apoptosis of Pro-T1, -T2, and -T3 cells correlates with Bcl-2 and Bax levels and is independent of Fas and p53 pathways. J Immunol [Internet] 1998; 160:5735–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9637482; PMID:963748214668867 [PubMed] [Google Scholar]
- 10.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsemeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature [Internet] 2003; 426:671-6. Available from:http://www.nature.com/nature/journal/v426/n6966/abs/nature02166.html; PMID:14668867; http://dx.doi.org/ 10.1038/nature02067 [DOI] [PubMed] [Google Scholar]
- 11.Li WQ, Jiang Q, Khaled AR, Keller JR, Durum SK. Interleukin-7 inactivates the pro-apoptotic protein Bad promoting T cell survival. J Biol Chem [Internet] 2004; 279:29160-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15123689; PMID:15123689; http://dx.doi.org/ 10.1074/jbc.M401656200 [DOI] [PubMed] [Google Scholar]
- 12.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naïve cells into memory-phenotype cells. Nat Immunol 2011; 12:478-84; PMID:21739670; http://dx.doi.org/ 10.1038/ni.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science 1999; 286:1381-3; PMID:10558997; http://dx.doi.org/ 10.1126/science.286.5443.1381 [DOI] [PubMed] [Google Scholar]
- 14.Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 1999; 286:1377-81; PMID:10558996; http://dx.doi.org/ 10.1126/science.286.5443.1377 [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 1998; 8:591-9; PMID:9620680; http://dx.doi.org/ 10.1016/S1074-7613(00)80564-6 [DOI] [PubMed] [Google Scholar]
- 16.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science (80-) 2000; 288:675-8; PMID:10784451; http://dx.doi.org/ 10.1126/science.288.5466.675 [DOI] [PubMed] [Google Scholar]
- 17.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR et al.. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med [Internet] 2000; 191:771-80. Available from:http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2195858&tool=pmcentrez&rendertype=abstract; PMID:10704459; http://dx.doi.org/ 10.1084/jem.191.5.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med [Internet] 2002; 195:1541-8. Available from: http://www.jem.org/cgi/doi/10.1084/jem.20020369; PMID:12070282; http://dx.doi.org/ 10.1084/jem.20020369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan JT. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med [Internet] 2002; 195:1523-32. Available from: http://www.jem.org/cgi/doi/10.1084/jem.20020066; PMID:12070280; http://dx.doi.org/ 10.1084/jem.20020066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol 2004; 22:765-87; PMID:15032596; http://dx.doi.org/ 10.1146/annurev.immunol.22.012703.104554 [DOI] [PubMed] [Google Scholar]
- 21.Stonier SW, Schluns KS. Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. Immunol Lett [Internet] 2010; 127:85-92. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2808451&tool=pmcentrez&rendertype=abstract; PMID:19818367; http://dx.doi.org/ 10.1016/j.imlet.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stonier SW, Ma LJ, Castillo EF, Schluns KS. Dendritic cells drive memory CD8 T-cell homeostasis via IL-15 transpresentation. Blood [Internet] 2008; 112:4546-54. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2597127&tool=pmcentrez&rendertype=abstract; PMID:18812469; http://dx.doi.org/ 10.1182/blood-2008-05-156307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramsey C, Rubinstein MP, Kim DM, Cho J, Sprent J, Surh CD. The lymphopenic environment of CD132 (common gamma chain)-deficient hosts elicits rapid homeostatic proliferation of naive T cells via IL-15. J Immunol 2008; 180:5320-6; PMID:18390713; http://dx.doi.org/ 10.4049/jimmunol.180.8.5320 [DOI] [PubMed] [Google Scholar]
- 24.Kieper WCW, Troy A, Burghardt JT, Ramsey C, Lee JY, Jiang H-Q, Dummer W, Shen H, Cebra JJ, Surh CD. Cutting edge: recent immune status determines the source of antigens that drive homeostatic T cell expansion. J Immunol [Internet] 2005; 174:3158-63. Available from:http://www.jimmunol.org/content/174/6/3158.abstract\nhttp://www.jimmunol.org/content/174/6/3158.short; PMID:15749843; http://dx.doi.org/ 10.4049/jimmunol.174.6.3158 [DOI] [PubMed] [Google Scholar]
- 25.Fry TJ, Connick E, Falloon J, Lederman MM, Liewehr DJ, Spritzler J, Steinberg SM, Wood LV, Yarchoan R, Zuckerman J et al.. A potential role for interleukin-7 in T-cell homeostasis. Blood [Internet] 2001; 97:2983-90. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11342421; PMID:11342421; http://dx.doi.org/ 10.1182/blood.V97.10.2983 [DOI] [PubMed] [Google Scholar]
- 26.Sheikh V, Porter BO, DerSimonian R, Kovacs SB, Thompson WL, Perez-Diez A, Freeman AF, Roby G, Mican J, Pau A et al.. Administration of interleukin-7 increases CD4 T cells in idiopathic CD4 lymphocytopenia. Blood [Internet] 2015; 127:977-88. Available from: http://www.bloodjournal.org/content/127/8/977.abstract; PMID:26675348; http://dx.doi.org/ 10.1182/blood-2015-05-645077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P, Labidi I et al.. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res 2009; 69:5383-91; PMID:19549917; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM et al.. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science (80- ) 2002; 298:850-4; PMID:12242449; http://dx.doi.org/ 10.1126/science.1076514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA et al.. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 2005; 23:2346-57; PMID:15800326; http://dx.doi.org/ 10.1200/JCO.2005.00.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest [Internet] 2002; 110:185-92. Available from:http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12122110\nhttp://www.jci.org/articles/view/15175/files/pdf?disposition=attachment; PMID:12122110; http://dx.doi.org/ 10.1172/JCI0215175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao J, Zhao L, Wan YY, Zhu B. Mechanism of action of IL-7 and its potential applications and limitations in cancer immunotherapy. Int J Mol Sci 2015; 16:10267-80; PMID:25955647; http://dx.doi.org/ 10.3390/ijms160510267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sportès C, Babb RR, Krumlauf MC, Hakim FT, Steinberg SM, Chow CK, Brown MR, Fleisher TA, Noel P, Maric I et al.. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin Cancer Res 2010; 16:727-35; PMID:20068111; http://dx.doi.org/16699374 10.1158/1078-0432.CCR-09-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg SA, Sportès C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, Maryalice Stetler-Stevenson KEM, Mavroukakis SA, Morre M, Buffet R et al.. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother 2006; 29:313-9; PMID:16699374; http://dx.doi.org/ 10.1097/01.cji.0000210386.55951.c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK et al.. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med [Internet] 2008; 205:1701-14. Available from:http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2442646/pdf/jem2051701.pdf; PMID:18573906; http://dx.doi.org/ 10.1084/jem.20071681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamoto Y, Douek DC, McFarland RD, Koup RA. Effects of exogenous interleukin-7 on human thymus function. Blood 2002; 99:2851-8; PMID:11929775; http://dx.doi.org/ 10.1182/blood.V99.8.2851 [DOI] [PubMed] [Google Scholar]
- 36.Suzuki T, Kishimoto H, Abe R. Requirement of interleukin 7 signaling for anti-tumor immune response under lymphopenic conditions in a murine lung carcinoma model. Cancer Immunol Immunother [Internet] 2016; 65:341-54. Available from: http://link.springer.com/10.1007/s00262-016-1808-7; PMID:26880265; http://dx.doi.org/ 10.1007/s00262-016-1808-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mueller K, Schweier O, Pircher H. Efficacy of IL-2- vs. IL-15-stimulated CD8 T cells in adoptive immunotherapy. Eur J Immunol [Internet] 2008; 38:2874-85. Available from: http://doi.wiley.com/10.1002/eji.200838426; PMID:18825743; http://dx.doi.org/ 10.1002/eji.200838426 [DOI] [PubMed] [Google Scholar]
- 38.Steel JC, Ramlogan CA, Yu P, Sakai Y, Forni G, Waldmann TA, Morris JC. Interleukin-15 and its receptor augment dendritic cell vaccination against the neu oncogene through the induction of antibodies partially independent of CD4 help. Cancer Res [Internet] 2010; 70:1072-81. Available from: http://cancerres.aacrjournals.org/content/70/3/1072.full; PMID:20086176; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaiser AD, Gadiot J, Guislain A, Blank CU. Mimicking homeostatic proliferation in vitro generates T cells with high anti-tumor function in non-lymphopenic hosts. Cancer Immunol Immunother 2013; 62:503-15; PMID:23001162; http://dx.doi.org/ 10.1007/s00262-012-1350-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadelain M. CAR therapy: The CD19 paradigm. J Clin Invest 2015; 125:3392-400; PMID:26325036; http://dx.doi.org/ 10.1172/JCI80010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maus MV, June CH. Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy. Clin Cancer Res [Internet] 2016; 22:1875-84. Available from: http://clincancerres.aacrjournals.org/content/22/8/1875.abstract; PMID:27084741; http://dx.doi.org/ 10.1158/1078-0432.CCR-15-1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, Heslop HE, Rooney CM, Brenner MK, Dotti G. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia [Internet] 2010; 24:1160-70. Available from:http://www.ncbi.nlm.nih.gov/pubmed/20428207; PMID:20428207; http://dx.doi.org/ 10.1038/leu.2010.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.North BYRJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med 1982; 55:1063-74; PMID:6460831; http://dx.doi.org/22933631 10.1084/jem.155.4.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Campion A, Pommier A, Delpoux A, Stouvenel L, Auffray C, Martin B, Lucas B. IL-2 and IL-7 determine the homeostatic balance between the regulatory and conventional CD4+ T cell compartments during peripheral T cell reconstitution. J Immunol [Internet] 2012; 189:3339-46. Available from:http://www.ncbi.nlm.nih.gov/pubmed/22933631; PMID:22933631; http://dx.doi.org/ 10.4049/jimmunol.1103152 [DOI] [PubMed] [Google Scholar]
- 45.Bolton HA, Zhu E, Terry AM, Guy TV, Koh WP, Tan SY, Power CA, Bertolino P, Lahl K, Sparwasser T et al.. Selective Treg reconstitution during lymphopenia normalizes DC costimulation and prevents graft-versus-host disease. J Clin Invest 2015; 125:3627-41; PMID:26301814; http://dx.doi.org/ 10.1172/JCI76031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peffault de Latour R, Dujardin HC, Mishellany F, Burlen-Defranoux O, Zuber J, Marques R, Di Santo J, Cumano A, Vieira P, Bandeira A. Ontogeny, function, and peripheral homeostasis of regulatory T cells in the absence of interleukin-7. Blood [Internet] 2006; 108:2300-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16763207; PMID:16763207; http://dx.doi.org/ 10.1182/blood-2006-04-017947 [DOI] [PubMed] [Google Scholar]
- 47.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ et al.. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science (80-) [Internet] 2013; 342:971-6. Available from: http://science.sciencemag.org/content/342/6161/971.abstract; PMID:24264990; http://dx.doi.org/ 10.1126/science.1240537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten Ra, Molina Da, Salcedo R, Back T, Cramer S et al.. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science (80-) [Internet] 2013; 342:967-70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24264989; PMID:24264989; http://dx.doi.org/ 10.1126/science.1240527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abu Eid R, Razavi GSE, Mkrtichyan M, Janik J, Khleif SN. Old-school chemotherapy in immunotherapeutic combination in cancer, a low-cost drug repurposed. Cancer Immunol Res [Internet] 2016; 4:377-82. Available from:http://cancerimmunolres.aacrjournals.org/cgi/doi/10.1158/2326-6066.CIR-16-0048; PMID:27196429; http://dx.doi.org/ 10.1158/2326-6066.CIR-16-0048 [DOI] [PubMed] [Google Scholar]
- 50.Machiels JH, Reilly RT, Emens LA, Vaccines W, Tolerized H-, Ercolini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice cyclophosphamide, doxorubicin, and paclitaxel enhance. Cancer Res 2001; 61:3689-97; PMID:11325840 [PubMed] [Google Scholar]


