Fig. 4. hiPSC-derived osteoblasts (Ad-hiPSCs) contribute to the healing of critical-sized bone defects through the formation of vascularized neobone tissue.
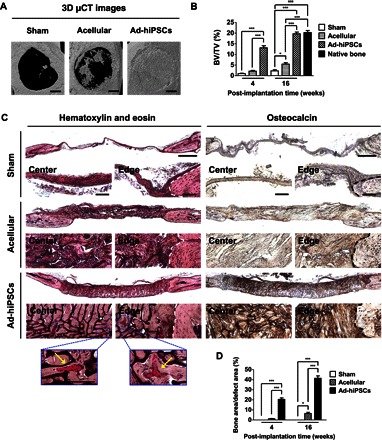
(A) Three-dimensional μCT images of cranial bone defects with no implantation (Sham), as well as defects treated with acellular and Ad-hiPSC–laden matrices [Ad-hiPSCs; hiPSC derivatives generated by culturing in growth medium supplemented with adenosine (30 μg/ml) for 21 days] after 16 weeks. Scale bars, 1 mm. (B) Bone mineral densities [bone volume per total volume (BV/TV)] of defects treated with acellular and Ad-hiPSC–laden matrices, as well as sham group following 4 and 16 weeks of implantation. Native mouse cranial bone was used as a control. (C) H&E staining and immunohistochemical staining for osteocalcin of cranial bone defects treated with acellular and Ad-hiPSCs–laden matrices, and sham group after 16 weeks. Scale bars, 500 μm. High-magnification images show the center and edge of cranial bone defects. White and black dashed lines mark the interface between newly formed tissue and the native bone tissue. Yellow arrows in H&E staining denote microvessels containing red blood cells. Scale bars, 100 μm. (D) Histomorphometric analysis for neobone density within cranial bone defects (Bone area/defect area) determined from H&E staining images for Sham, Acellular, and Ad-hiPSCs groups at 4 and 16 weeks after treatment. Data are shown as means ± SEs (n = 6). Multiple groups at the same post-implantation time were compared by one-way ANOVA with Tukey-Kramer post hoc test. Asterisks were assigned to P values with statistical significances (*P < 0.05; ***P < 0.001).
